Prof. S. Winawer (chair, USA)
Prof. M. Classen (co-chair, Germany)
Prof. R. Lambert(co-chair, France)
Prof. M. Fried (Switzerland)
Prof. P. Dite (Czech Republic)
Prof. K.L. Goh (Malaysia)
Prof. F. Guarner (Spain)
Prof. D. Lieberman (USA)
Prof. R. Eliakim (Israel)
Prof. B. Levin (USA)
Prof. R. Saenz (Chile)
Prof. A.G. Khan (Pakistan)
Prof. I. Khalif (Russia)
Prof. A. Lanas (Spain)
Prof. G. Lindberg (Sweden)
Prof. M.J. O’Brien (USA)
Prof. G. Young (Australia)
Dr. J. Krabshuis (France)
Prof. R. Smith (USA)
Prof. W. Schmiegel (Germany)
Prof. D. Rex (USA)
Prof. N. Amrani (Morocco)
Prof. A. Zauber (USA)
1. Introduction
Colorectal cancer (CRC) is a worldwide problem, with an annual incidence of approximately 1 million cases and an annual mortality of more than 500,000. The absolute number of cases will increase over the next two decades as a result of the aging and expansion of populations in both the developed and developing countries.
CRC is the second most common cause of cancer mortality among men and women. Most CRCs arise from sporadic adenomas, and a few from genetic polyposis syndromes or inflammatory bowel disease (IBD). The term “polyp” refers to a discrete mass that protrudes into the intestinal lumen. The reported prevalence of adenomatous polyps, on the basis of screening colonoscopy data, is in the range of 18–36%.
The risk for CRC varies from country to country and even within countries. The risk also varies among individual people based on diet, lifestyle, and hereditary factors.
The most common neoplastic outcome of colorectal cancer screening is the adenoma. After removal, patients need to be placed in a follow-up surveillance program, as do the patients with identified and treated cancer.
These guidelines are directed to screening: the testing of asymptomatic men and women who are likely to have adenomatous polyps or cancer. Screening needs to be applied within the framework of a program that includes: primary prevention (diet, lifestyle), timely diagnostic work-up with colonoscopy (where available and consistent with the cascade) in those screened positive, and timely treatment (polypectomy, surgery).
Colorectal cancer screening is particularly challenging, as reflected in current low screening rates in most countries where there is a high risk for colorectal cancer. Colorectal cancer screening is complex, as there are multiple options, it requires considerable patient effort (fecal occult blood test slides, colonoscopy preparation, etc.), and it requires sedation and a health-care partner for some tests (colonoscopy). For a screening program to be successful, multiple events have to occur, beginning with awareness and recommendation from the primary-care physician, patient acceptance, financial coverage, risk stratification, screening test, timely diagnosis, timely treatment, and appropriate follow-up. If any one of these steps is faulty or is not of high quality, the screening will fail.
2. Methodology and literature review
WGO guidelines summarize what is known and has been published in existing systematic reviews, evidence-based guidelines, and high-quality trials, and this information is then configured to make the guideline as relevant and accessible as possible globally. Usually, this means building different approaches in order to achieve the same ends — each approach is different because it attempts to take into account local resources, cultural preferences, and policies. WGO guidelines are not systematic reviews based on a systematic and comprehensive review of all the available evidence and guidelines. A global guideline tries to distinguish between areas with differing resources and differing epidemiologies, and the guideline is then translated into different languages to facilitate relevance and access.
This guideline was written by the review team following a series of literature searches to establish what had changed since the WGO’s first position statement on the topic of colorectal cancer screening, published in 2002 (http://omge.org/globalguidelines/statement03/statement3.htm).
The available evidence was searched using a precise rather than sensitive syntax for each platform searched. Relevant guidelines were searched in the United States National Guideline Clearinghouse platform at www.ngc.org and on the web sites of the major medical societies concerned with gastroenterology and cancer. Further searches were carried out in Medline and EMBASE on the Dialog-Datastar platform for 2003 onwards. A search in the Cochrane Library yielded 18 relevant systematic reviews and 12 protocols. The review team members were each assigned specific sections in accordance with their own expertise and preferences. The team’s librarian supported each section team with dedicated searches for further back-up and detail. Finally, international experts were consulted for each section written by the review team, and the entire draft was edited by the review team chair and the librarian.
3. Epidemiology of colorectal cancer
3.1 The burden of colorectal cancer
In the Globocan 2002 database of the International Agency for Research on Cancer (IARC), the worldwide burden of colorectal cancer is estimated as 550,000 incident new cases and 278,000 deaths for men, and 473,000 incident new cases and 255,000 deaths for women. In 2002, colorectal cancer comprised 9.4% of the global cancer burden in both sexes and was most frequent in North America, Australia, New Zealand, and parts of Europe. This has led to colorectal cancer being considered as a disease of the Western lifestyle.
3.2 Temporal trends in incidence and mortality
Age-standardized rates (ASR) of mortality from colorectal cancer in men and women in Western countries remained stable throughout the 20th century, and may now be starting to decline; on the other hand, rapid changes are being experienced in countries previously considered to be at low risk.
In Europe, age-standardized mortality rates have increased in eastern and southern Europe, while leveling off in most northern and central European countries. In recent years, mortality trends have tended to be systematically more favorable for females than males.
In the USA, trends in the incidence rates of colorectal cancer in the Surveillance Epidemiology and End Results (SEER) registries suggest that between 1973 and 1989, the age-standardized incidence of colon cancer in men rose by 11% in whites and 39% in blacks, whereas the incidence of rectal cancer fell by 5% in whites and rose by 27% in blacks. In women, colon cancer incidence declined by 3% in whites and increased by 26% in blacks, whereas rectal cancer rates fell by 7% and 10%, respectively. Since 1990, the age-standardized incidence rates of colon cancer have been declining. The practice of prevention by polypectomy may have played a role in this.
In Japan, the age-standardized mortality rates for colorectal cancer were low in the mid-20th century and increased approximately threefold in both sexes between the time periods of 1955–74 and 1975–84.
With the world’s population aging, a considerable increase in the number of cases is to be expected.
3.3 Familial and genetic factors in colorectal cancer
Fig. 1 Familial risk factors and colorectal cancer
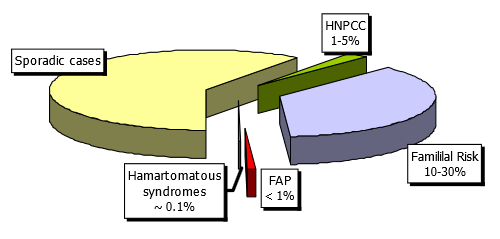
Average risk. The risk of CRC increases with age and family history. Colorectal cancer is rare before the age of 50, but after that threshold, the incidence of CRC increases dramatically. Those with no family history are considered to be at average risk.
Nonsyndromic familial risk. CRC is perhaps the most familial of all human cancers. The estimated proportion of colorectal cancers that is attributable to heritable causes varies from 5% to 30%. Inherited syndromes with known genetic defects account only for 1–5% of all CRCs. Between 10% and 30% of CRC patients have a familial history of CRC but do not belong to a known inherited syndrome. Familial clustering of cases is common and appears to confer increased risk. First-degree relatives of persons with CRC have a twofold to threefold increase in the risk of CRC in comparison with control or population incidence. Moreover, the risk increases with the number of relatives with CRC, the closer the relatives are to the patient, and with the age of CRC in family members. Individuals with a personal history of colorectal cancer are also at increased risk for subsequent development of cancer. Thus, the risk of CRC is increased in persons with a family history of nonsyndromic CRC and in those with a history of adenomas in close relatives under the age of 60 (Table 1).
Table 1 Familial risk of colorectal cancer

RR, relative risk; CI, confidence intervals.
Adapted from: Burt RW (Gastroenterol Clin North Am 1996;25:793–803) and Johns LE, Houlston RS (Am J Gastroenterol 2001;96:2992–3003).
Syndromic familial risk. Familial adenomatous polyposis (FAP). FAP is autosomal-dominant. One-third of new cases are caused by a de novo mutation.
Hereditary nonpolyposis colorectal cancer (HNPCC) or Lynch syndrome. HNPCC with autosomal-dominant transmission is the most common form of syndromic familial colorectal cancer. A consensus group has established a list of criteria (the Amsterdam II criteria) that suggest the presence of the HNPCC phenotype (Table 2).
Table 2 Amsterdam II criteria for hereditary nonpolyposis colorectal cancer (HNPCC)

Other less common familial syndromes are:
- Juvenile polyposis (JP)
- Peutz–Jeghers syndrome (PJ)
- Cowden syndrome
Clinical risk factors in inflammatory bowel disease. The increased risk of developing colorectal cancer in patients with IBD is well established. The cumulative incidence of cancer starts to increase approximately 8–10 years after the onset of the disease and rises to 15% by 30 years. Risk factors include long duration of the disease, extent of the disease, young age at onset, and the presence of complicating primary sclerosing cholangitis or stenotic disease. Inadequate pharmacological therapy (possibly) and lack of adequate surveillance can also pose an additional risk.
4. Screening tests and evidence, 1: stool tests, occult blood, and DNA
Colorectal cancers may shed blood and other tissue components that can be detected in the feces long before the development of clinical symptoms. This has led to a search for stool analyses that can allow early detection of cancer and screening for colorectal cancer in people without symptoms. The most common method has been the detection of occult blood in feces. This has been shown in several randomized studies to reduce the mortality of colorectal cancer by 15–33% in the cohorts and by 45% in the compliers, depending on the type of slide and frequency of testing.
Occult blood tests: The guaiac smear test is the most common test for detecting fecal occult blood. The guaiac test reacts to the peroxidase activity of heme, but this makes the test liable to reaction with other peroxidases in the feces, such as those from certain fruits, vegetables, and red meat. Dietary restrictions are therefore necessary to avoid false-positive results.
There are several problems with the fecal occult blood test (FOBT) as a screening test for colorectal cancer. The sensitivity of the test is only 50–60% for one-time use, but may be as high as 90% when it is used every 1–2 years over a long period of time (programmatic sensitivity). Although the sensitivity can be increased using rehydration, this leads to variability in the reaction that invalidates the method as a screening procedure. Low sensitivity leads to a high number of false-negative results and the effect of false reassurance. The majority of cases identified by fecal occult blood testing are false-positive, and these patients will be subjected to unnecessary further investigations, usually colonoscopy. Another problem with FOBT screening is that its effectiveness requires compliance with testing over many years.
Fecal occult blood testing using the guaiac smear is currently being replaced in many countries by fecal immunochemical tests (FIT or IFOBT), which detect hemoglobin using sensitive and specific techniques. Such tests obviate the need for dietary restrictions. The user-friendliness of the tests varies; some are more user-friendly and have very good compliance. The optimum cut-off point for sensitivity in the immunochemical tests remains to be validated.
Fecal DNA tests for colorectal cancer. It has been suggested that the identification of abnormal DNA in stool samples may provide a possible method for early detection of colorectal cancer. However, the optimal set of molecular markers remains to be determined, and the feasibility of such tests when applied to the general population is as yet unknown. One study compared a panel of 21 mutations against fecal occult blood determined by the standard guaiac smear test in 2507 subjects. The sensitivity of the DNA panel for colorectal cancer was 52%, in comparison with 13% with the guaiac smear test, and the specificity was similar (94.4% vs. 95.2%). The results for the guaiac test were extremely low.
Several additional small studies have been conducted, yielding an aggregate sensitivity of 65% and specificity of 95%. A new version with fewer mutations has brought the sensitivity to over 80%.
5. Screening tests and evidence, 2: endoscopy and CT colonography
This section describes endoscopic and radiographic procedures used to screen for colorectal cancer and the evidence underlying their use.
5.1 Endoscopic screening procedures
Flexible sigmoidoscopy allows direct examination of the inner surface of the large bowel up to a distance of about 60 cm from the anal margin. This technique can detect colorectal polyps and cancers and is also used to remove polyps or take tissue samples for histological examination. The advantages of flexible sigmoidoscopy are that the procedure can be performed by physician and nonphysician examiners; it is less time-consuming than colonoscopy; bowel preparation is also easier and quicker; the morbidity is negligible in examinations that do no not require polypectomy; and no sedation is needed. However, its obvious disadvantage is that examination of the left colon alone misses right-sided lesions. While the specificity of the findings with the endoscopic procedure is very high (98–100%, few false-positives), the sensitivity is low for the entire colon and ranges from 35% to 70% due to the significant number of right-sided adenomas that occur in the absence of distal tumors and are therefore missed on flexible sigmoidoscopy.
Sigmoidoscopy is being used for screening in asymptomatic individuals for early cancer detection and prevention. Case–control studies have clearly shown that screening sigmoidoscopy decreases colon cancer mortality by 60–70% in the area examined. Major complications occur in one per 10,000 cases.
Colonoscopy allows the detection and removal of polyps and biopsy of cancer throughout the colon. Both the specificity and sensitivity of colonoscopy for detecting polyps and cancer are high (at least 95% for large polyps; see below). The miss rate for polyps, on the basis of studies of back-to-back colonoscopies, is 15–25% for adenomas smaller than 5 mm in diameter and 0–6% for adenomas of 10 mm or more.
There are no prospective randomized studies that have examined the impact of colonoscopy on incidence or mortality. However, long-term follow-up of postpolypectomy patients in the United States National Polyp Study demonstrated an approximately 90% reduction in the incidence and mortality of colorectal cancer, using mathematical modeling.
Ideally, a screening procedure should be a simple and inexpensive test that could easily be applied to the entire at-risk population. While these criteria are not fulfilled by colonoscopy, this approach is the “gold standard,” and patients with a positive result on any other screening test (FOBT, sigmoidoscopy, computed-tomographic colonography) should be referred subsequently for colonoscopy if it is available. In some countries in which resources are available, direct colonoscopy has become the most prevalent procedure for CRC screening. Major complications occur in 1–2 per 1000 cases.
5.2 Radiographic screening procedures
Double-contrast barium enema. Although double-contrast barium enema (DCBE) allows evaluation of the entire colon, its sensitivity and specificity are inferior to those of colonoscopy and computed-tomographic colonography. Even for large polyps and cancers, DCBE offers substantially lower sensitivity (48%) than colonoscopy, and DCBE is more likely than colonoscopy to yield false-positives (artifacts diagnosed as polyps). Patients with an abnormal barium enema need a subsequent colonoscopy. However, DCBE is widely available, and the fact that it may detect up to 50% of large polyps would support the use of this procedure in patients in the absence of other resources.
Computed-tomographic colonography (CTC). Thin-section helical computed-tomographic scanning of the abdomen and pelvis, followed by digital processing and interpretation of the images, can display two-dimensional and three-dimensional reconstructions of the colonic lumen (“virtual colonoscopy”). The procedure requires air insufflation for colonic distension to maximal tolerance (approximately 2 L of room air or carbon dioxide) and cathartic bowel preparation. Ingestion of oral contrast can “tag” fecal material and fluid, which can then be digitally subtracted from the image on the computer.
A meta-analysis of studies using CTC for the detection of colorectal polyps and cancer showed high sensitivity (93%) and high specificity (97%) levels with this technique for polyps 10 mm or larger. However, for large and medium-sized polyps combined (6 mm or larger), the average sensitivity decreased to 86%, with a specificity of 86%. When polyps of all sizes were included, the studies were too heterogeneous in sensitivity (range 45–97%) and specificity (range 26–97%). While the sensitivity of CTC for cancer and large polyps is satisfactory, detection of polyps in the 6–9-mm size range is not satisfactory. An important drawback of CTC for screening patients at increased risk is that flat lesions are missed.
A major disadvantage of CTC for its use as a screening procedure is the repeated exposure of patients to ionizing radiation. Recently, multidetector or multislice CT technology has shortened the scan time and reduced the radiation dose, while maintaining high spatial resolution. Magnetic resonance colonography is being studied in Europe for this reason.
In addition, the issue of when to refer patients for colonoscopy is unresolved on the basis of the polyp size visualized on CTC. This has an enormous impact on the cost of the screening. Another disadvantage is that the examination requires a complete bowel preparation. If patients need colonoscopy, they have to undergo a second bowel preparation unless facilities exist to do both on the same day. Finally, extraintestinal findings can lead to additional radiologic and surgical evaluation and increased costs. Major complications are rare.
6. Cost-effectiveness of CRC screening
All standard options for CRC screening in average-risk individuals are cost-effective. They are as cost-effective as mammography and more cost-effective than other forms of medical screening (e.g., for cholesterol in hypertension). Systematic screening colonoscopy in first-degree relatives of patients with CRC, starting at the age of 40, demonstrates an economic benefit. In comparison with multiple-drug intensive chemotherapy for advanced cancer, screening is cost-saving.
7. Cascades – tooling up for screening
7.1 Introduction
Different screening options for average-risk and higher-risk men and women aged 50 and over are reviewed here. The options take account of the availability of colonoscopy, flexible sigmoidoscopy, FOBT, and barium enema. When screening resources are severely limited, the most realistic option would be fecal occult blood testing every year or two for average-risk men and women, starting at the age of 50.
The type of slide test used depends on screening resources and the dietary habits of the population.
Lower test positivity with Hemoccult II will tax colonoscopy resources less than more sensitive slide tests such as Hemoccult SENSA. Immunochemical tests are optimal, in that they require only two rather than three days of testing and require no dietary restrictions, but they cost more, which is a consideration when financial resources are low.
The diagnostic work-up can be with either colonoscopy, if available, or barium enema if colonoscopy is not readily available. Thus, the decision to identify separately people who are at increased risk depends on the colonoscopy resources available. If these are very limited, then people who are at increased risk can be screened along with average-risk people.
7.2 CRC screening cascade
The CRC screening cascade consists of a set of recommendations. The recommendations apply to different resource levels, beginning with 1 (highest resources) and ending with 6 (minimal resources available).
Cascade level 1. The recommendations below are appropriate for countries with a relatively high level of resources (financial, professional, facilities) where the colorectal cancer incidence and mortality is high (IARC data) and is an important concern relative to other public health priorities.
Recommendations for screening people at average risk. Colonoscopy for average-risk men and women, starting at the age of 50 and every 10 years in the absence of factors that would place them at increased risk.
Recommendations for screening people at increased risk:
- — People with a family history of colorectal cancer or adenomatous polyps.
— People with a first-degree relative (parent, sibling, or child) with colon cancer or adenomatous polyps diagnosed under the age of 60, or with two first-degree relatives diagnosed with colorectal cancer at any age, should be advised to have screening colonoscopy starting at the age of 40, or 10 years younger than the earliest diagnosis in their family, whichever comes first, and repeated every 5 years.
— People with a first-degree relative with a colon cancer or adenomatous polyp diagnosed when he or she was over the age of 60, or with two second-degree relatives with colorectal cancer, should be advised to be screened as average-risk persons, but starting at the age of 40.
— People with one second-degree relative (grandparent, aunt, or uncle) or third-degree relative (great-grandparent or cousin) with colorectal cancer should be advised to be screened as average-risk persons.
- Familial adenomatous polyposis (FAP). People who have a genetic diagnosis of familial adenomatous polyposis, or who are at risk of having FAP but in whom genetic testing has not been performed or is not feasible, should have an annual sigmoidoscopy, beginning at age 10–12, to determine whether they are expressing the genetic abnormality. Genetic testing should be considered in patients with FAP who have relatives at risk. Genetic counseling should guide genetic testing and consideration of colostomy.
- Hereditary nonpolyposis colorectal cancer (HNPCC). People with a genetic or clinical diagnosis of hereditary nonpolyposis colorectal cancer, or who are at increased risk for HNPCC, should have colonoscopy every 1–2 years, starting at the age of 20–25 or 10 years earlier than the youngest age of colon cancer diagnosis in the family, whichever comes first. Genetic testing for HNPCC should be offered to first-degree relatives of persons with a known inherited mismatch repair (MMR) gene mutation. It should also be offered when the family mutation is not already known, but one of the first three of the modified Bethesda criteria is met.
- People with a history of inflammatory bowel disease or a history of adenomatous polyps or colorectal cancer are candidates for follow-up surveillance, rather than screening. Guidelines have been published for the surveillance of these individuals.
Cascade level 2. The recommendations are the same as for level 1, but they apply when colonoscopy resources are more limited.
Recommendations for screening people at average risk. Colonoscopy for average-risk men and women at age 50 once in a lifetime, in the absence of factors that would place them at increased risk.
Recommendations for screening people at increased risk. Recommendations for screening people who are at increased risk are the same as for cascade 1.
Cascade level 3. The recommendations are the same as for level 1, but they apply when the colonoscopy resources are more limited and flexible sigmoidoscopy resources are available.
Recommendations for screening people at average risk. Flexible sigmoidoscopy for average-risk men and women, starting at the age of 50, every 5 years, in the absence of factors that would place them at increased risk. Diagnostic work-up with colonoscopy for positive sigmoidoscopy.
Recommendations for screening people at increased risk. Recommendations for screening people at increased risk are the same as for level 1.
Cascade level 4. The recommendations are the same as for level 3, but they apply when the flexible sigmoidoscopy and colonoscopy resources are more limited.
Recommendations for screening people at average risk. Flexible sigmoidoscopy for average-risk men and women once in a lifetime at the age of 50, in the absence of factors that would place them at increased risk. Diagnostic colonoscopy work-up for positive sigmoidoscopy or advanced neoplasia, depending on the available colonoscopy resources.
Recommendations for screening people at increased risk. Recommendations for screening people at increased risk are the same as for level 1.
Cascade level 5. The recommendations are the same as for resource level 4, but they apply when diagnostic colonoscopy is severely limited.
Recommendations for screening people at average risk. Flexible sigmoidoscopy for average-risk men and women once in a lifetime at the age of 50. Diagnostic colonoscopy only if advanced neoplasia is detected.
Recommendations for screening people at increased risk. The recommendations for screening people at increased risk depend on the colonoscopic resources available.
Cascade level 6. The recommendations are the same as for level 1, but they apply when colonoscopy and flexible sigmoidoscopy resources are severely limited.
Recommendations for screening people at average risk. Fecal blood testing every year for average-risk men and women starting at the age of 50, in the absence of factors that would place them at increased risk. The type of test used depends on colonoscopy resources and the dietary habits of the population. Diagnostic work-up can be either with colonoscopy, if available, or barium enema if colonoscopy is not readily available.
Recommendations for screening people at increased risk. The decision to separately identify these people for special screening (see level 1) depends on the available colonoscopy resources. If not available, these people can be screened along with average-risk individuals.
7. 3 New tests
CTC and DNA testing are available only in a few high-resource countries and are generally not applicable globally. However, where available, they can be offered to average-risk men and women, starting at the age of 50, who do not wish to be screened by other more standard methods, in order to increase the low number of people currently being screened in these countries.
7.4 Recommendations for action — implementing a program
Recommendations for action — general:
- Develop and disseminate structured educational programs for members of the public, providers, health-care systems, and policy-makers/political leaders. Effective educational programs should be directed to each of the important participants in an acceptable manner.
- Develop evidence-based standards for quality throughout the screening process.
- Develop and disseminate inexpensive, easy-to-use clinical management systems.
- Advocate screening through national and local venues.
- Promote colorectal cancer screening as part of comprehensive clinical preventive care.
Recommendations for action — program design
Planning the screening program:
- A target population should be identified — i.e., asymptomatic men and women, age, risk factors (e.g., familial).
- The decision to implement colorectal cancer screening should be based on the relative burden of colorectal cancer in the population to be screened.
- The screening strategy (test, interval, age range) should be based on medical evidence (guidelines), availability of resources, level of risk, and cultural acceptance by the population.
- Support by influential professional and patient advocacy groups and from the media is essential.
- Evaluate the feasibility of the proposed program. Address the development and allocation of resources (financial, personnel, facilities).
- Evaluate the specific cultural and language needs of the population.
Implementing the screening program:
- Identify the target unit for implementation, and ensure communication (training and education) with providers (general practitioners and others) and the target population.
- Develop and disseminate guidelines on screening, diagnosis, treatment, and surveillance in a patient-friendly and culturally sensitive manner.
- Develop methods for initial patient enrollment and follow-up.
Monitoring the screening program:
- Careful, timely monitoring of the following rates: screening uptake, re-screening, and follow-up of positive tests.
- Compliance with surveillance recommendations.
- Measurement of the program quality should be in place, and evaluated regularly.
- Outcomes, including detection rates, cancer stage distribution, adenoma detection, complications, and, finally, the effect on the population incidence and mortality.
8. Where to get help
8.1 IDCA
- http://omge.org/?idca The International Digestive Cancer Alliance (IDCA)
The mission of the International Digestive Cancer Alliance is to promote the prevention and management of digestive cancers worldwide through an international alliance of organizations that share the same goal.
8.2 International Agency for Research on Cancer (IARC)
The IARC is part of the World Health Organization. The main emphasis of its research is on epidemiology, environmental carcinogenesis, and research training.
8.3 United States Centers for Disease Control and Prevention (CDC)
The CDC site has the best overall information on screening, which is available free of charge at:
8.4 ACS American Cancer Society (ACS)
Together with the National Comprehensive Cancer Network (NCCN), the ACS has produced excellent treatment guidelines on colon and rectal cancer:
8.5 Union Internationale Contre le Cancer (International Union Against Cancer, UICC)
As the world’s largest independent, non-profit, nongovernmental association of cancer-fighting organizations, the UICC is a catalyst for responsible dialogue and collective action. The UICC brings together a wide range of organizations, including voluntary cancer societies, research and treatment centers, public health authorities, patient support networks, and advocacy groups.
9. Useful web sites, guidelines and selected references
9.1 Guidelines, consensus statements, web sites
Abbreviations: ACG, American College of Gastroenterology; ACS, American Cancer Society; AGA, American Gastroenterological Association; ASCRS, American Society of Colorectal Surgeons; ASGE, American Society of Gastrointestinal Endoscopy; BSG, British Society of Gastroenterology; DGVS, German Society for Digestive and Metabolic Diseases; FMSD, Finnish Medical Society Duodecim; ICSI, Institute for Clinical Systems Improvement; NCCN, National Comprehensive Cancer Network; NICE, National Institute of Clinical Excellence; NZGG, New Zealand Guidelines Group; SIGN, Scottish Intercollegiate Guidelines Network; SMH, Singapore Ministry of Health; WGO, World Gastroenterology Organisation.
9.2 Further reading
- Lieberman DA, Weiss DG; Veterans Affairs Cooperative Study Group 380. One-time screening for colorectal cancer with combined fecal occult-blood testing and examination of the distal colon. N Engl J Med 2001;345:555–60 (PMID: 11529208).
- Barclay RL, Vicari JJ, Doughty AS, Johanson JF, Greenlaw RL. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med 2006;355:2533–41 (PMID: 17167136).
- Sonnenberg A, Delcò F, Inadomi JM. Cost-effectiveness of colonoscopy in screening for colorectal cancer. Ann Intern Med 2000;133:573–84 (PMID: 11033584).
- Burt R, Neklason DW. Genetic testing for inherited colon cancer. Gastroenterology 2005;128:1696–1716 (PMID: 15887160).
- Faivre J, Dancourt V, Lejeune C, Tazi MA, Lamour J, Gerard D, et al. Reduction in colorectal cancer mortality by fecal occult blood screening in a French controlled study. Gastroenterology 2004;126):1674–80 (PMID: 15188160).
- Imperiale TF, Ransohoff DF, Itzkowitz SH, Turnbull BA, Ross ME. Fecal DNA versus fecal occult blood for colorectal-cancer screening in an average-risk population. N Engl J Med 2004;351:2704–14 (PMID: 15616205).
- Kronborg O, Jorgensen OD, Fenger C, Rasmussen M. Randomized study of biennial screening with a fecal occult blood test: results after nine screening rounds. Scand J Gastroenterol 2004;39:846–51 (PMID: 15513382).
- Parkin DM, Whelan SL, Ferlay J, et al., editors. Cancer incidence in five continents, vol. 8. Lyons: International Agency for Research on Cancer, 2002 (IARC Scientific Publications, no. 155) (PMID: 12812229).
- Winawer S, Fletcher R, Rex D, Bond J, Burt R, Ferrucci J, et al. Colorectal cancer screening and surveillance: clinical guidelines and rationale — update based on new evidence. Gastroenterology 2003;124:544–60 (PMID: 12557158).
- Winawer SJ, Zauber AG, Fletcher RH, Stillman JS, O’Brien MJ, Levin B, et al. Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. Gastroenterology 2006;130:1872–85 (PMID: 16697750).
- Young GP, St. John DJ, Winawer SJ, Rozen P; WHO (World Health Organization) and OMED (World Organization for Digestive Endoscopy). Choice of fecal occult blood tests for colorectal cancer screening: recommendations based on performance characteristics in population studies: a WHO (World Health Organization) and OMED (World Organization for Digestive Endoscopy) report. Am J Gastroenterol 2002;97:2499–507 (PMID: 12385430).
- Hewitson P, Glasziou P, Irwig L, Towler B, Watson E. Screening for colorectal cancer using the faecal occult blood test, Hemoccult. Cochrane Database Syst Rev 2007;(1):CD001216 (PMID: 17253456).
- Regula J, Rupinski M, Kraszewska E, Polkowski M, Pachlewski J, Orlowska J, et al. Colonoscopy in colorectal-cancer screening for detection of advanced neoplasia. N Engl J Med 2006;355:1863–72 (PMID: 17079760).
- Winawer SJ, Zauber AG, Ho MN, O’Brien MJ, Gottlieb LS, Sternberg SS, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med 1993;329:1977–81 (PMID: 8247072).
- Winawer SJ, Stewart ET, Zauber AG, Bond JH, Ansel H, Waye JD, et al. A comparison of colonoscopy and double-contrast barium enema for surveillance after polypectomy. National Polyp Study Work Group. N Engl J Med 2000;342:1766–72 (PMID 10852998).
- Winawer SJ, Zauber AG, Gerdes H, O’Brien MJ, Gottlieb LS, Sternberg SS, et al. Risk of colorectal cancer in the families of patients with adenomatous polyps. National Polyp Study Workgroup. N Engl J Med 1996;334:82–7 (PMID: 8531963).
- Itzkowitz-Steven-H, Jandorf-Lina, Brand-Randall, Rabeneck-Linda, Schroy-Paul-C-3rd, Sontag-Stephen, Johnson-David, Skoletsky-Joel, Durkee-Kris, Markowitz-Sanford, Shuber- Anthony. Improved fecal DNA test for colorectal cancer screening. Clinical gastroenterology and hepatology Jan 2007 08 Dec 2006 , vol. 5, no. 1, p.111-7 PMID: 17161655
- Kim-David-H, Pickhardt-Perry-J, Taylor-Andrew-J, Leung-Winifred-K, Winter-Thomas-C, Hinshaw-J-Louis, Gopal-Deepak-V, Reichelderfer-Mark, Hsu-Richard-H, Pfau-Patrick-R. CT colonography versus colonoscopy for the detection of advanced neoplasia. N Engl J Med, 4 Oct 2007, vol. 357, no. 14, p. 1403-12, PMID: 17914041
- Allison JE, Sakoda LC, Levin TR, Tucker JP, Tekawa IS, Cuff T, Pauly MP, Shlager L, Palitz AM, Zhao WK, Schwartz JS, Ransohoff DF, Selby JV. Screening for colorectal neoplasms with new fecal occult blood tests: update on performance characteristics. J Natl Cancer Inst. 2007 Oct 3;99(19):1462-70. PMID: 17895475
We are grateful to the International Agency for Research on Cancer (IARC; www.iarc.fr for data and tables, and to Annals of Oncology (http://annonc.oxfordjournals.org/) for data from the Oslo meeting.
10. Queries and feedback
The Practice Guidelines Committee welcomes any comments and queries that readers may have. Do you feel we have neglected some aspects of the topic? Do you think that some procedures are associated with extra risk? Tell us about your own experience. You are welcome to click on the link below and let us know your views.
guidelines@worldgastroenterology.org







