This World Gastroenterology Organisation (WGO) guideline on ‘Endoscope Disinfection’ is intended for use by health providers and professionals who are involved in the use, cleaning, and maintenance of endoscopes, and it aims to support national societies, official bodies, and individual endoscopy departments in developing local standards and protocols for reprocessing endoscopes.
These WGO Guidelines are the result of a systematic development process for expert consensus based on the medical and scientific literature, existing practice guidelines, and (regional) best-practice standards. The update addresses the recent outbreaks of multidrug-resistant organisms after endoscopy and proposes measures to reduce the risks of these outbreaks occurring. The recommendations are based on the consensus findings of an international multidisciplinary working group with expertise in microbiology, including biofilms, endoscope reprocessing, nursing, and gastroenterology, and with broad experience in developing national and international reprocessing guidelines.
1.1 Guidelines or standards
The delivery of safe and effective endoscopic services is governed by overlapping national and international standards, including those for the design and staffing of the facilities, automatic flexible endoscope reprocessors, disinfectants, water quality, and drying cabinets.
The implementation of the appropriate standards for reprocessing should follow the general principles of good manufacturing practice (GMP). GMP is a set of regulations, codes, and guidelines for a manufacturing process — in this case, reprocessing an endoscope — to produce high-level disinfection, which covers both the process and quality control. GMP is recognized worldwide for the control and management of manufacturing and quality control testing of pharmaceutical products and has evolved over the last 60 years in response to multiple well-publicized problems in the pharmaceutical industry [1].
Reprocessing instructions are often called “guidelines,” but are in fact a technical standard that sets out the minimum acceptable practice for reprocessing to deliver high-level disinfection of endoscopes. Medical guidelines usually address a narrow clinical question using population-based data — often the results of randomized trials — to guide the care of an individual patient. Randomized trials are performed in specific populations, and clinicians must decide if the guidelines are applicable to their individual patient [2].
Standards are broader in application and set out specifications and procedures that are designed to ensure that products, services, and systems are safe, reliable, and consistently perform in the way they were intended. The supporting evidence for a standard is based on science, technology, and experience. Randomized trials in a specific population are rarely performed. The standards governing reprocessing are based on science and are often validated by measurements of efficiency in models with artificial soils or a known inoculum of bacteria. The science of cleaning, disinfection, drying, and microbiology forms the basis of reprocessing standards relevant in all countries.
Standards set out the minimum acceptable practice.
The terms “guidelines” and “standards” are both used to describe instructions for endoscope reprocessing [3,4].
1.2 General principles in endoscope reprocessing
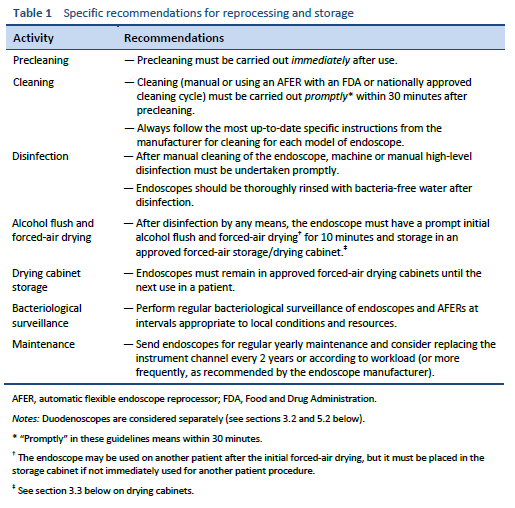
The most important step in endoscope reprocessing is scrupulous manual cleaning prior to disinfection. Disinfection will fail if cleaning has been inadequate [5–7].
Manual cleaning must be undertaken by a person familiar with the structure of the endoscope and trained in cleaning technique. Cleaning should begin immediately after the endoscope is used, so that biological material does not dry and harden. Appropriate detergents and cleaning equipment should be used, and in particular brushes with the appropriate diameter should be used for each channel. Cleaning should be followed by thorough rinsing to ensure that all debris and detergents are removed prior to disinfection.
1.2.1 Manual cleaning
Precleaning: immediately after each procedure, with the endoscope still attached to the light source, wipe the insertion tube with a lint-free disposable cloth. Place the distal tip in a low-foaming medical-grade detergent solution and aspirate detergent through all the channels, including the suction/biopsy channel. Flush the air/water channels with detergent. Flush all channels, including the jet channel if present, with water, then air, as per the manufacturer’s instructions. Flushing of the air/water channels with detergent may require the use of a specific valve.
Remove the endoscope from the light source and transport it to the cleaning area in a closed container that avoids environmental contamination from dripping or spillage and that clearly indicates that the endoscope within is contaminated.
It is essential that the endoscope is not allowed to dry prior to further cleaning, as this will make removal of organic matter difficult or impossible. Endoscopes should be processed without delay, within 30 minutes.
Leak testing should be performed to check the integrity of all channels before further processing. Remove all the valves and buttons and leak-test the instrument as per the manufacturer’s instructions.
Brush and clean buttons and valves, paying particular attention to internal surfaces, and carry out high-level disinfection or sterilization according to the original equipment manufacturer’s instructions.
Place the endoscope in a detergent solution in a sink in the “dirty” section of the decontamination area and wash its outer surface. A low-foaming medical-grade detergent should be used at the appropriate dilution according to the manufacturer’s instructions. Brush all accessible sections of the suction biopsy channel according to the manufacturers’ instructions for use. Each channel should be brushed until all debris is removed. Brush the tip and handles and clean valve seats. Fit cleaning adaptors and flush channels with fresh detergent for the product-specified time.
The endoscope should be rinsed by draining the detergent from the sink, rinsing the outer surface with cold running tap water, and then filling the sink with tap water and purging the channels with tap water, using the cleaning adapters following the manufacturer’s instructions. Purge the channels with air to remove rinse water.
1.2.2 Disinfection
High-level disinfection is performed in an automatic flexible endoscope reprocessor (AFER), which should comply with the relevant national standard or be approved by the U.S. Food and Drug Administration (FDA). The AFER may or may not have an automated cleaning cycle as well as the disinfection cycle. All connectors should be specifically designed for each endoscope model. Ensure that all channels are connected at the start and end of a cycle. The detachable components, including the air/water and suction valves, can be steam-sterilized or reprocessed with the endoscope if the ability of the AFER to clean and/or disinfect these detachable components is validated by the AFER manufacturer.
After high-level disinfection, the endoscope is rinsed in the AFER with bacteria-free water produced by submicron filters. Water quality should be checked regularly.
Manual high-level disinfection is another option that is effective when performed by well-trained, dedicated reprocessing staff supplied with the appropriate personal protective equipment. The endoscope is immersed in disinfectant, and all channels are filled with disinfectant solution. Immerse the buttons and valves in the disinfectant. Soak the instrument for the required time at the required temperature and concentration as specified by the disinfectant manufacturer.
Purge the disinfectant from all channels with air and rinse the exterior of the endoscope and flush the channels with bacteria-free water, with the volume required for the specific disinfectant used, to remove any traces of disinfectant.
1.2.3 Drying
Endoscopes should be dried after each procedure by purging the water from the channels with compressed air, then flushing the channels with alcohol, followed by forced-air drying. Alcohol flushing facilitates drying and is a useful adjunct to disinfection, due to its bactericidal effects [8].
The use of alcohol may not be permitted in some countries (France, UK) due to concerns about variant Creutzfeldt–Jakob disease (CJD).
The endoscope is then stored in a forced-air drying cabinet to supplement drying.
If an endoscope is used infrequently, it is reasonable to store it separately, hanging vertically in a purpose-built cabinet, as opposed to a forced-air storage/drying cabinet, and to reprocess the endoscope prior to the next patient use. Endoscopes should be dried completely prior to hanging.
1.2.4 Accessories
The water bottle should be changed after each endoscopy session and steam-sterilized. The water bottle should be filled with sterile water immediately prior to use.
1.2.5 Documentation
All essential steps of endoscope reprocessing should be documented for quality assurance and for patient tracing if necessary.
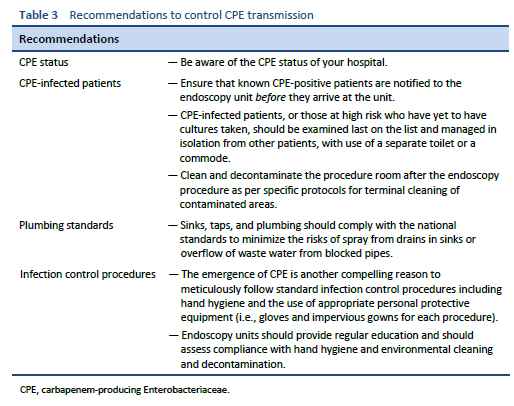
During outbreaks of MDROs after endoscopy, patients may become colonized with bacteria and initially show no clinical symptoms, only to develop serious systemic infections weeks to months later, with mortality rates reported to be as high as 40% [36,52].
Often, a single species of CPE is transmitted from one endoscope on multiple occasions, despite reprocessing. This epidemiology is best explained by a biofilm on the endoscope that protects bacteria from cleaning and disinfection and acts as a reservoir for the transmission of infection.
5.1 Biofilm
In the 1999 Centers for Disease Control and Prevention (CDC) report of an outbreak of a carbapenemase-producing Pseudomonas aeruginosa following bronchoscopy, it was considered that biofilm formation in difficult-to-clean narrow endoscopy channels contributed to the outbreak [53]. A subsequent research investigation examined the surfaces of endoscope channels using scanning electron microscopy and confirmed the presence of biofilm, often lodged in surface defects [32]. Other studies have also found biofilm on endoscope channels [54–56] and on culprit endoscopes in reports of outbreaks [57–59].
Biofilm is a community of bacteria that are attached to a surface and to each other by an extracellular polysaccharide matrix. Bacteria living in a biofilm have properties different from those of free-floating (planktonic) bacteria of the same species. Bacteria incorporated into biofilms are resistant to disinfectants used at recommended reprocessing concentrations [60]. Planktonic CPEs are killed in under 1 minute by standard disinfectants, providing a wide safety margin for these planktonic bacteria [61]. However, the biofilm matrix limits the diffusion of the disinfectant, and multiple layers of cells and biofilm matrix are difficult for the disinfectant to penetrate [62]. Standard concentrations of disinfectants do not reliably kill the same bacteria within biofilms [63]. Bacteria in build-up biofilm (BBF) that accumulates in defects on endoscope channel surfaces are also protected by organic debris and cross-linked protein, making them more difficult to kill with standard reprocessing [31,55]. Current reprocessing parameters are based on data from models that use artificial soils and planktonic bacteria rather than models incorporating bacteria in biofilm or BBF.
Biofilm acts as a reservoir of bacteria attached to the surface of an endoscope channel, and given favorable conditions, bacteria in biofilms can multiply, detach, resume their planktonic state, and be transmitted to patients during endoscopy [31]. Moisture and a supply of nutrients facilitate biofilm growth and the release of planktonic bacteria.
The role of moisture in facilitating biofilm growth during storage and the importance of complete drying after reprocessing have been underestimated in the past. Current evidence indicates that 95% of endoscopes still had visible moisture in channels after AFER alcohol flush, a 3-minute drying cycle, and overnight storage in a regular cabinet [64]. Keeping the endoscope free of moisture — particularly the channels during storage — must be a priority.
Biofilm readily forms on endoscope defects, often longitudinal wear marks on biopsy channels, and is difficult or impossible to remove with standard reprocessing [31,32,55,65]. A multicenter study of patient-ready endoscopes found defects in all 45 endoscopes examined [66]. Channel inspection with a borescope commonly identifies occult surface defects [66–68]. Endoscopes should have regular maintenance to identify and repair macroscopic defects and routine channel replacement to reduce the prevalence of occult defects and help maintain smooth, cleanable channel surfaces [6,37]. The investigation by Verfallie et al. of the culprit duodenoscope in an outbreak found that duodenoscope O-rings were an important area of concern; these should be replaced annually together with the channels [36]. Other endoscopes may require less frequent replacement of channels, perhaps 1–2-yearly depending on the workload.
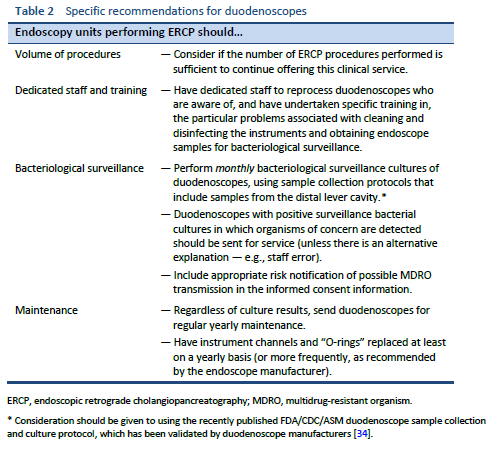
5.2 Duodenoscopes
Duodenoscopes are difficult to clean and disinfect. In addition to their complex design, factors such as the characteristics of patients referred for ERCP and the interventions performed also contribute to the risk of colonization and subsequent infection from bacteria transmitted during the procedure.
The rate of contamination of duodenoscopes, as judged by positive surveillance cultures, is similar to the rates of contamination of gastroscopes and colonoscopes [18–21]. Thus, patient characteristics and the interventions performed are dominant factors in the higher incidence of outbreaks after ERCP.
The risks of outbreaks are best addressed by specific changes to improve cleaning and disinfection of duodenoscopes, as well as improvements to reprocessing for all endoscopes. The manufacturers’ updated cleaning protocols are an important improvement in duodenoscope reprocessing. A review of a quality-assurance database of 4307 duodenoscope cultures found that implementation of the new cleaning protocols significantly reduced the rate of positive cultures [69].
5.3 Drying
The reprocessing step of drying has often been ignored or carried out incompletely, and it is prone to human error [37]. A survey in the United States of reprocessing in 249 endoscopy units performing ERCPs found that 52% of the centers did not comply with the multisociety guidelines and did not use forced air to dry endoscopes [70]. Guidelines are inconsistent with one another and do not always specify the parameters for adequate drying [71]. Recent studies have found residual fluid in up to 95% of endoscope channels after reprocessing and drying, suggesting that drying guidelines need improvement [55,64].
Biofilms need moisture to grow. Alfa and Sitter, in a pivotal paper, demonstrated that if duodenoscopes were left damp after reprocessing, there was rapid growth of Pseudomonas and Acinetobacter species [72]. Drying for 10 minutes with forced air prevented this overgrowth in all duodenoscopes studied. Implementation of an alcohol flush followed by forced-air drying ended outbreaks of Pseudomonas infections following ERCP in the 1980s [73]. More recent studies have confirmed that alcohol flushing followed by 10 minutes of forced-air drying was more effective than alcohol flushing followed by a shorter, variable time of forced-air drying [66,74].
The Association for periOperative Registered Nurses (AORN) guidelines [4] recommend that endoscopes should be stored in a drying cabinet and state “The collective evidence shows that optimal storage of flexible endoscopes facilitates drying, decreases the potential for contamination, and provides protection from environmental contaminants.”
This recommendation is supported by a review of surveillance cultures of patient-ready endoscopes, including duodenoscopes, gastroscopes, colonoscopes and echoendoscopes, which found that the introduction of drying cabinets significantly reduced the risk of endoscope contamination [75]. In a direct comparison, a forced-air drying cabinet dried endoscopes more rapidly and significantly reduced microbial growth in comparison with a standard storage cabinet [76].
5.4 Simethicone
Simethicone is a silicone-based polymer used in endoscopy to improve visibility. Randomized trials confirm a decrease in the number of bubbles and improved visibility. However, simethicone is not water-soluble, and in 2009 Olympus warned that simethicone was difficult to remove with standard reprocessing [77]. In 2016, van Stiphout et al. reported that if simethicone was added to water injected via the colonoscope’s water-jet channel, crystal deposits formed in the water-jet connector and channel [78]. A more recent study has confirmed that various concentrations of simethicone flushed down the biopsy/suction channel form a residue that is not completely removed by standard reprocessing [79]. Residual simethicone may interfere with drying and increase the risk of biofilm formation, which may result in microbes surviving high-level disinfection and sterilization. In June 2018, Olympus recommended against the use of simethicone and other non–water-soluble substances [80]. A recent editorial notes that both Pentax and FujiFilm also recommend against using simethicone with their endoscopes, and the authors advise that endoscope manufacturers’ instructions should be followed [81].
5.5 Tropical infections
There is very little evidence available on the risk of transmission of parasitic infections by gastrointestinal endoscopy. To become infective, most parasitic agents require progression in a life cycle that takes time, so that they are not immediately infective. Most potentially infective parasites would not survive endoscope reprocessing.
There is generally considered to be no risk with respect to helminths, nematodes, platyhelminths, Anisakis, or liver flukes such as Fasciola hepatica, but there is one report of four cases of Strongyloides esophagitis related to a single gastroscope [82]. However, concerns have been raised with regard to the risk of transmission of Giardia lamblia, Cryptosporidium species, and amebas.
5.6 Conclusion
The science of reprocessing is evolving. New research — including basic research, clinical research, and randomized trials that have been undertaken in response to published reports of outbreaks of CPE — is now being published. Endoscope manufacturers are continuing to improve endoscope design and validate new reprocessing instructions. New drying and cleaning technologies are emerging into the marketplace. Professional societies are producing updated versions of reprocessing guidelines in response to the flood of information.
This guideline, along with other recent guidelines, recommends that hospitals appoint a multidisciplinary committee with a diversity of interests and expertise to assess new information as it is published and to develop, implement, and — importantly — regularly update reprocessing guidelines that are appropriate to the hospital’s resources and patient mix.
Effective reprocessing is key to patient safety in endoscopy.
1. Patel KT, Chotai NP. Pharmaceutical GMP: past, present, and future—a review. Pharmazie. 2008;63(4):251–5.
2. Evidence-Based Medicine Working Group. Evidence-based medicine. A new approach to teaching the practice of medicine. JAMA. 1992;268(17):2420–5.
3. Society of Gastroenterology Nurses and Associates (SGNA). Standards and position statements [Internet] [Internet]. Chicago, IL: Society of Gastroenterology Nurses and Associates (SGNA); 2019 [cited 2018 Jan 18]. Available from: https://www.sgna.org/Practice/Standards-Practice-Guidelines
4. Association for periOperative Registered Nurses (AORN). Guidelines for perioperative practice [Internet]. Denver, CO: AORN, Inc.; 2019. Available from: https://www.aornbookstore.org/Product/Detail/MAN019
5. Gastroenterological Society of Australia (GESA). Infection control in endoscopy [Internet] [Internet]. Melbourne: Gastroenterological Society of Australia (GESA); 2010 [cited 2018 Jan 18]. Available from: http://www.gesa.org.au/resources/infection-control-in-endoscopy/
6. Kenters N, Huijskens E, Meier C, Voss A. Infectious diseases linked to cross-contamination of flexible endoscopes. Endosc Int Open. 2015;3(4):E259–65.
7. Alfa MJ. Current issues result in a paradigm shift in reprocessing medical and surgical instruments. Am J Infect Control. 2016 May;44(5):e41–5.
8. Kovacs BJ, Chen YK, Kettering JD, Aprecio RM, Roy I. High-level disinfection of gastrointestinal endoscopes: are current guidelines adequate? Am J Gastroenterol. 1999;94(6):1546–50.
9. Hayes, Inc. FDA Advisory Panel offers recommendations on procedures for reprocessing duodenoscopes [press release] [Internet]. Dallas, TX: Hayes, Inc.; 2015 [cited 2018 Feb 7]. Available from: https://www.hayesinc.com/hayes/resource-center/news-service/HNS-20150420-49/
10. U.S. Food and Drug Administration (FDA). Division of Industry and Consumer Education (DICE). Infections associated with reprocessed flexible bronchoscopes: FDA safety communication [Internet]. Silver Spring, MD: U.S. Food and Drug Administration (FDA); 2015 [cited 2018 Feb 9]. Available from: http://wayback.archive-it.org/7993/20170722213119/https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm462949.htm
11. Naas T, Cuzon G, Babics A, Fortineau N, Boytchev I, Gayral F, et al. Endoscopy-associated transmission of carbapenem-resistant Klebsiella pneumoniae producing KPC-2 beta-lactamase. J Antimicrob Chemother. 2010;65(6):1305–6.
12. Bajolet O, Ciocan D, Vallet C, de Champs C, Vernet-Garnier V, Guillard T, et al. Gastroscopy-associated transmission of extended-spectrum beta-lactamase-producing Pseudomonas aeruginosa. J Hosp Infect. 2013 Apr;83(4):341–3.
13. Orsi GB, García-Fernández A, Giordano A, Venditti C, Bencardino A, Gianfreda R, et al. Risk factors and clinical significance of ertapenem-resistant Klebsiella pneumoniae in hospitalised patients. J Hosp Infect. 2011;78(1):54–8.
14. Koo VSW, O’Neill P, Elves A. Multidrug-resistant NDM-1 Klebsiella outbreak and infection control in endoscopic urology. BJU Int. 2012;110(11 Pt C):E922-926.
15. Tumbarello M, Spanu T, Sanguinetti M, Citton R, Montuori E, Leone F, et al. Bloodstream infections caused by extended-spectrum-beta-lactamase-producing Klebsiella pneumoniae: risk factors, molecular epidemiology, and clinical outcome. Antimicrob Agents Chemother. 2006;50(2):498–504.
16. Orsi GB, Bencardino A, Vena A, Carattoli A, Venditti C, Falcone M, et al. Patient risk factors for outer membrane permeability and KPC-producing carbapenem-resistant Klebsiella pneumoniae isolation: results of a double case-control study. Infection. 2013;41(1):61–7.
17. Voor In ’t Holt AF, Severin JA, Hagenaars MBH, de Goeij I, Gommers D, Vos MC. VIM-positive Pseudomonas aeruginosa in a large tertiary care hospital: matched case-control studies and a network analysis. Antimicrob Resist Infect Control. 2018;7:32.
18. Bisset L, Cossart YE, Selby W, West R, Catterson D, O’Hara K, et al. A prospective study of the efficacy of routine decontamination for gastrointestinal endoscopes and the risk factors for failure. Am J Infect Control. 2006;34(5):274–80.
19. Brandabur JJ, Leggett JE, Wang L, Bartles RL, Baxter L, Diaz GA, et al. Surveillance of guideline practices for duodenoscope and linear echoendoscope reprocessing in a large healthcare system. Gastrointest Endosc. 2016;84(3):392-399.e3.
20. Saliou P, Héry-Arnaud G, Le Bars H, Payan C, Narbonne V, Cholet F, et al. Evaluation of current cleaning and disinfection procedures of GI endoscopes. Gastrointest Endosc. 2016;84(6):1077.
21. Jones D. [Australia’s microbiological surveillance experience.]. In: U.S. Food and Drug Administration (FDA). Center for Devices and Radiological Health. Medical Devices Advisory Committee. Gastroenterology and Urology Devices Panel, editor. [Transcript of meeting held on May 14, 2015, Silver Spring, Maryland] [Internet]. Silver Spring, MD: U.S. Food and Drug Administration (FDA); 2015 [cited 2019 May 31]. p. 142–5. Available from: https://wayback.archive-it.org/7993/20170113091355/http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/MedicalDevices/MedicalDevicesAdvisoryCommittee/Gastroenterology-UrologyDevicesPanel/UCM451164.pdf
22. Kelly CR, Kahn S, Kashyap P, Laine L, Rubin D, Atreja A, et al. Update on fecal microbiota transplantation 2015: indications, methodologies, mechanisms, and outlook. Gastroenterology. 2015;149(1):223–37.
23. Cammarota G, Ianiro G, Tilg H, Rajilić-Stojanović M, Kump P, Satokari R, et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut. 2017;66(4):569–80.
24. Rutala WA. ERCP scopes: a need to shift from disinfection to sterilization? In: U.S. Food and Drug Administration (FDA). Center for Devices and Radiological Health. Medical Devices Advisory Committee. Gastroenterology and Urology Devices Panel, editor. [Transcript of meeting held on May 15, 2015, Silver Spring, Maryland] [Internet]. Silver Spring, MD: U.S. Food and Drug Administration (FDA); 2015 [cited 2018 Mar 6]. p. 307–18. Available from: https://wayback.archive-it.org/7993/20170113091400/http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/MedicalDevices/MedicalDevicesAdvisoryCommittee/Gastroenterology-UrologyDevicesPanel/UCM451165.pdf
25. U.S. Food and Drug Administration (FDA). Gastroenterology-Urology Devices Panel. 2015 materials of the Gastroenterology-Urology Devices Panel [Internet] [Internet]. Silver Spring, MD: U.S. Food and Drug Administration (FDA); 2015 [cited 2018 Feb 9]. Available from: https://wayback.archive-it.org/7993/20170112002249/http:/www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/MedicalDevices/MedicalDevicesAdvisoryCommittee/Gastroenterology-UrologyDevicesPanel/ucm445590.htm
26. Petersen BT, Cohen J, Hambrick RD, Buttar N, Greenwald DA, Buscaglia JM, et al. Multisociety guideline on reprocessing flexible GI endoscopes: 2016 update. Gastrointest Endosc. 2017;85(2):282-294.e1.
27. Snyder GM, Wright SB, Smithey A, Mizrahi M, Sheppard M, Hirsch EB, et al. Randomized comparison of 3 high-Level disinfection and sterilization procedures for duodenoscopes. Gastroenterology. 2017;153(4):1018–25.
28. U.S. Food and Drug Administration (FDA). Division of Industry and Consumer Education (DICE). Supplemental measures to enhance duodenoscope reprocessing: FDA safety communication [Internet]. Silver Spring, MD: U.S. Food and Drug Administration (FDA); 2015 [cited 2018 Feb 9]. Available from: http://wayback.archive-it.org/7993/20170722150658/https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm454766.htm
29. Thaker AM, Kim S, Sedarat A, Watson RR, Muthusamy VR. Inspection of endoscope instrument channels after reprocessing using a prototype borescope. Gastrointest Endosc. 2018;88(4):612–9.
30. Bartles RL, Leggett JE, Hove S, Kashork CD, Wang L, Oethinger M, et al. A randomized trial of single versus double high-level disinfection of duodenoscopes and linear echoendoscopes using standard automated reprocessing. Gastrointest Endosc. 2018;88(2):306–313.e2.
31. Alfa MJ, Ribeiro MM, da Costa Luciano C, Franca R, Olson N, DeGagne P, et al. A novel polytetrafluoroethylene-channel model, which simulates low levels of culturable bacteria in buildup biofilm after repeated endoscope reprocessing. Gastrointest Endosc. 2017 Sep;86(3):442-451.e1.
32. Pajkos A, Vickery K, Cossart Y. Is biofilm accumulation on endoscope tubing a contributor to the failure of cleaning and decontamination? J Hosp Infect. 2004;58(3):224–9.
33. Roberts CG. The role of biofilms in reprocessing medical devices. Am J Infect Control. 2013;41(5 Suppl):S77-80.
34. U.S. Food and Drug Administration, Centers for Disease Control and Prevention (CDC), American Society for Microbiology (ASM). Duodenoscope surveillance. Sampling and culturing: reducing the risks of infection [Internet]. Silver Spring, MD: U.S. Food and Drug Administration (FDA); 2018 [cited 2018 Mar 7]. 58 p. Available from: https://www.fda.gov/downloads/medicaldevices/productsandmedicalprocedures/reprocessingofreusablemedicaldevices/ucm597949.pdf
35. Weingarten RA, Johnson RC, Conlan S, Ramsburg AM, Dekker JP, Lau AF, et al. Genomic analysis of hospital plumbing reveals diverse reservoir of bacterial plasmids conferring carbapenem resistance. mBio. 2018;9(1):e02011-17.
36. Verfaillie CJ, Bruno MJ, Voor in ’t Holt AF, Buijs JG, Poley J-W, Loeve AJ, et al. Withdrawal of a novel-design duodenoscope ends outbreak of a VIM-2-producing Pseudomonas aeruginosa. Endoscopy. 2015;47(6):493–502.
37. Jung M, Beilenhoff U. Hygiene: the looming Achilles heel in endoscopy. Visc Med. 2016;32(1):21–8.
38. Ling ML, Ching P, Widitaputra A, Stewart A, Sirijindadirat N, Thu LTA. APSIC guidelines for disinfection and sterilization of instruments in health care facilities. Antimicrob Resist Infect Control. 2018;7:25.
39. Murdani A, Kumar A, Chiu H-M, Goh K-L, Jang BI, Khor CJL, et al. WEO position statement on hygiene in digestive endoscopy: focus on endoscopy units in Asia and the Middle East. Dig Endosc. 2017;29(1):3–15.
40. Beilenhoff U, Neumann C, Rey J, Biering H, Blum R, Schmidt V. ESGE-ESGENA guideline for quality assurance in reprocessing: Microbiological surveillance testing in endoscopy. Endoscopy. 2007;39(02):175–81.
41. Chinese Society of Digestive Endoscopy. Consensus of experts on the safe operation of digestive endoscopy centers in China. J Dig Dis. 2016;17(12):790–9.
42. Roberts G, Roberts C, Jamieson A, Grimes C, Conn G, Bleichrodt R. Surgery and obstetric care are highly cost-effective interventions in a sub-Saharan African district hospital: a three-month single-institution study of surgical costs and outcomes. World J Surg. 2016;40(1):14–20.
43. Rennert-May E, Conly J, Leal J, Smith S, Manns B. Economic evaluations and their use in infection prevention and control: a narrative review. Antimicrob Resist Infect Control. 2018;7:31.
44. Bartsch SM, McKinnell JA, Mueller LE, Miller LG, Gohil SK, Huang SS, et al. Potential economic burden of carbapenem-resistant Enterobacteriaceae (CRE) in the United States. Clin Microbiol Infect. 2017;23(1):48.e9-48.e16.
45. Bardossy AC, Zervos J, Zervos M. Preventing hospital-acquired infections in low-income and middle-income countries. Infect Dis Clin North Am. 2016 Sep;30(3):805–18.
46. Association for the Advancement of Medical Instrumentation (AAMI). ANSI/AAMI ST91:2015 Comprehensive guide to flexible and semi-rigid endoscope processing in health care facilities [Internet]. Arlington, VA: Association for the Advancement of Medical Instrumentation; 2015. Available from: https://www.aami.org/productspublications/ProductDetail.aspx?ItemNumber=2477
47. Association for the Advancement of Medical Instrumentation (AAMI). Preventing device-related healthcare-associated infections: issues and outcomes from the September 2016 forum, Medical Technology and HAIs [Internet]. Arlington, VA: Association for the Advancement of Medical Instrumentation (AAMI); 2016 [cited 2018 Feb 7]. 19 p. Available from: https://s3.amazonaws.com/rdcms-aami/files/production/public/FileDownloads/Summits/161227_AAMI_HAI_Forum_Report.pdf
48. International Organization for Standardization (ISO). ISO 9000:2015(en). Quality management systems — fundamentals and vocabulary [Internet]. Geneva: International Organization for Standardization (ISO); 2015 [cited 2018 Jan 18]. Available from: https://www.iso.org/obp/ui/#iso:std:iso:9000:ed-4:v1:en
49. International Organization for Standardization (ISO). ISO 9001:2015. Quality management systems — requirements [Internet]. Geneva: International Organization for Standardization (ISO); 2015 [cited 2018 Jan 18]. Available from: https://www.iso.org/standard/62085.html
50. International Organization for Standardization (ISO). ISO 13485:2016. Medical devices — quality management systems — requirements for regulatory purposes [Internet]. Geneva: International Organization for Standardization (ISO); 2016 [cited 2018 Jan 18]. Available from: https://www.iso.org/standard/59752.html
51. Beilenhoff U, Biering H, Blum R, Brljak J, Cimbro M, Dumonceau J-M, et al. Prevention of multidrug-resistant infections from contaminated duodenoscopes: position statement of the European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastroenterology Nurses and Associates (ESGENA). Endoscopy. 2017;49(11):1098–106.
52. Kallen AJ. CDC outbreak investigation. In: U.S. Food and Drug Administration (FDA). Center for Devices and Radiological Health. Medical Devices Advisory Committee. Gastroenterology and Urology Devices Panel, editor. [Transcript of meeting held on May 14, 2015, Silver Spring, Maryland] [Internet]. Silver Spring, MD: U.S. Food and Drug Administration (FDA); 2015 [cited 2018 Jun 3]. p. 199–210. Available from: https://wayback.archive-it.org/7993/20170113091355/http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/MedicalDevices/MedicalDevicesAdvisoryCommittee/Gastroenterology-UrologyDevicesPanel/UCM451164.pdf
53. Centers for Disease Control and Prevention (CDC). Bronchoscopy-related infections and pseudoinfections—New York, 1996 and 1998. MMWR Morb Mortal Wkly Rep. 1999;48(26):557–60.
54. Wu R-P, Xi H-J, Qi K, Wang D, Nie X, Li Z-S. Correlation between the growth of bacterial biofilm in flexible endoscopes and endoscope reprocessing methods. Am J Infect Control. 2014;42(11):1203–6.
55. Hervé RC, Keevil CW. Persistent residual contamination in endoscope channels; a fluorescence epimicroscopy study. Endoscopy. 2016;48(7):609–16.
56. Herrmann IF, Heeg P, Matteja B, Strahl HM, Werner H-P, Boyce W, et al. Risques et dangers cachés de l’endoscopie, conduite à tenir. Acta Endosc. 2008;38(5):493–502.
57. Buss A, Been M, Borgers R, Stokroos I, Melchers W, Peters F, et al. Endoscope disinfection and its pitfalls — requirement for retrograde surveillance cultures. Endoscopy. 2008;40(04):327–32.
58. Kovaleva J, Meessen N, Peters F, Been M, Arends J, Borgers R, et al. Is bacteriologic surveillance in endoscope reprocessing stringent enough? Endoscopy. 2009;41(10):913–6.
59. Johani K, Hu H, Santos L, Schiller S, Deva AK, Whiteley G, et al. Determination of bacterial species present in biofilm contaminating the channels of clinical endoscopes. Infect Dis Health. 2018;23(4):189–96.
60. Otter JA, Vickery K, Walker JT, deLancey Pulcini E, Stoodley P, Goldenberg SD, et al. Surface-attached cells, biofilms and biocide susceptibility: implications for hospital cleaning and disinfection. J Hosp Infect. 2015;89(1):16–27.
61. Olson J. Medivators. In: U.S. Food and Drug Administration (FDA). Center for Devices and Radiological Health. Medical Devices Advisory Committee. Gastroenterology and Urology Devices Panel, editor. [Transcript of meeting held on May 14, 2015, Silver Spring, Maryland] [Internet]. Silver Spring, MD: U.S. Food and Drug Administration (FDA); 2015 [cited 2018 Jun 3]. p. 69–77. Available from: https://wayback.archive-it.org/7993/20170113091355/http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/MedicalDevices/MedicalDevicesAdvisoryCommittee/Gastroenterology-UrologyDevicesPanel/UCM451164.pdf
62. Bridier A, Briandet R, Thomas V, Dubois-Brissonnet F. Resistance of bacterial biofilms to disinfectants: a review. Biofouling. 2011;27(9):1017–32.
63. Akinbobola AB, Sherry L, Mckay WG, Ramage G, Williams C. Tolerance of Pseudomonas aeruginosa in in-vitro biofilms to high-level peracetic acid disinfection. J Hosp Infect. 2017 Oct;97(2):162–8.
64. Ofstead CL, Wetzler HP, Johnson EA, Heymann OL, Maust TJ, Shaw MJ. Simethicone residue remains inside gastrointestinal endoscopes despite reprocessing. Am J Infect Control. 2016;44(11):1237–40.
65. da Costa Luciano C, Olson N, Tipple AFV, Alfa M. Evaluation of the ability of different detergents and disinfectants to remove and kill organisms in traditional biofilm. Am J Infect Control. 2016;44(11):e243–9.
66. Ofstead CL, Heymann OL, Quick MR, Eiland JE, Wetzler HP. Residual moisture and waterborne pathogens inside flexible endoscopes: Evidence from a multisite study of endoscope drying effectiveness. Am J Infect Control. 2018;46(6):689–96.
67. Ofstead CL, Doyle EM, Eiland JE, Amelang MR, Wetzler HP, England DM, et al. Practical toolkit for monitoring endoscope reprocessing effectiveness: identification of viable bacteria on gastroscopes, colonoscopes, and bronchoscopes. Am J Infect Control. 2016;44(7):815–9.
68. Ofstead CL, Wetzler HP, Eiland JE, Heymann OL, Held SB, Shaw MJ. Assessing residual contamination and damage inside flexible endoscopes over time. Am J Infect Control. 2016;44(12):1675–7.
69. Higa JT, Choe J, Tombs D, Gluck M, Ross AS. Optimizing duodenoscope reprocessing: rigorous assessment of a culture and quarantine protocol. Gastrointest Endosc. 2018;88(2):223–9.
70. Thaker AM, Muthusamy VR, Sedarat A, Watson RR, Kochman ML, Ross AS, et al. Duodenoscope reprocessing practice patterns in U.S. endoscopy centers: a survey study. Gastrointest Endosc. 2018;88(2):316-322.e2.
71. Kovaleva J. Endoscope drying and its pitfalls. J Hosp Infect. 2017;97(4):319–28.
72. Alfa MJ, Sitter DL. In-hospital evaluation of contamination of duodenoscopes: a quantitative assessment of the effect of drying. J Hosp Infect. 1991 Oct;19(2):89–98.
73. Petersen BT. Duodenoscope reprocessing: risk and options coming into view. Gastrointest Endosc. 2015;82(3):484–7.
74. Barakat MT, Huang RJ, Banerjee S. Comparison of automated and manual drying in the eliminating residual endoscope working channel fluid after reprocessing (with video). Gastrointest Endosc. 2018 Aug 24;
75. Saliou P, Le Bars H, Payan C, Narbonne V, Cholet F, Jézéquel J, et al. Measures to improve microbial quality surveillance of gastrointestinal endoscopes. Endoscopy. 2016;48(8):704–10.
76. Perumpail RB, Marya NB, McGinty BL, Muthusamy VR. Endoscope reprocessing: comparison of drying effectiveness and microbial levels with an automated drying and storage cabinet with forced filtered air and a standard storage cabinet [Epub ahead of print]. Am J Infect Control. 2019 Apr 6;
77. Catalone BJ, Olympus America Inc. Simethicone [letter to customers] [Internet]. 2009 Jun 9 [cited 2019 May 31]; Available from: https://medical.olympusamerica.com/sites/default/files/pdf/SimethiconeCustomerLetter.pdf
78. van Stiphout S, Laros I, van Wezel R, Gilissen L. Crystallization in the waterjet channel in colonoscopes due to simethicone. Endoscopy. 2016;48(S 01):E394–5.
79. Barakat MT, Huang RJ, Banerjee S. Simethicone is retained in endoscopes despite reprocessing: impact of its use on working channel fluid retention and adenosine triphosphate bioluminescence values (with video). Gastrointest Endosc. 2019;89(1):115–23.
80. ECRI Institute. Olympus—flexible endoscopes: manufacturer recommends against use of simethicone/non-water soluble additives — Alert [Internet]. Plymouth Meeting, PA: ECRI Institute; 2018 [cited 2019 Mar 1]. Available from: https://www.ecri.org/Components/Alerts/Pages/login.aspx?Page=AlertDisplay&AId=1635719
81. Visrodia K, Petersen BT. Borescope examination: Is there value in visual assessment of endoscope channels? Gastrointest Endosc. 2018;88(4):620–3.
82. Mandelstam P, Sugawa C, Silvis SE, Nebel OT, Rogers BH. Complications associated with esophagogastroduodenoscopy and with esophageal dilation. Gastrointest Endosc. 1976;23(1):16–9.