1. Bray GA, Frühbeck G, Ryan DH, Wilding JP. Management of obesity. Lancet (London, England) 2016;387: 1947-1956.
2. Rueda-Clausen CF, Poddar M, Lear A, Poirier P, Sharma AM. Canadian Adult Obesity Clinical Practice Guidelines: Assessment of People Living with Obesity 2020. Available from: https://obesitycanada.ca/guidelines/assessment/.
3. Abdullah A, Peeters A, de Courten M, Stoelwinder J. The magnitude of association between overweight and obesity and the risk of diabetes: a meta-analysis of prospective cohort studies. Diabetes Res Clin Pract 2010;89: 309-319.
4. Maggio CA, Pi-Sunyer FX. Obesity and type 2 diabetes. Endocrinol Metab Clin North Am 2003;32: 805-822, viii.
5. Piché ME, Tchernof A, Després JP. Obesity Phenotypes, Diabetes, and Cardiovascular Diseases. Circ Res 2020;126: 1477-1500.
6. van Dis I, Kromhout D, Geleijnse JM, Boer JM, Verschuren WM. Body mass index and waist circumference predict both 10-year nonfatal and fatal cardiovascular disease risk: study conducted in 20,000 Dutch men and women aged 20-65 years. Eur J Cardiovasc Prev Rehabil 2009;16: 729-734.
7. Ortega FB, Lavie CJ, Blair SN. Obesity and Cardiovascular Disease. Circ Res 2016;118: 1752-1770.
8. Powell-Wiley TM, Poirier P, Burke LE, Després JP, Gordon-Larsen P, Lavie CJ, et al. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021;143: e984-e1010.
9. Meurling IJ, Shea DO, Garvey JF. Obesity and sleep: a growing concern. Curr Opin Pulm Med 2019;25: 602-608.
10. Crummy F, Piper AJ, Naughton MT. Obesity and the lung: 2. Obesity and sleep-disordered breathing. Thorax 2008;63: 738-746.
11. Lakkis JI, Weir MR. Obesity and Kidney Disease. Prog Cardiovasc Dis 2018;61: 157-167.
12. Silva Junior GB, Bentes AC, Daher EF, Matos SM. Obesity and kidney disease. J Bras Nefrol 2017;39: 65-69.
13. Avgerinos KI, Spyrou N, Mantzoros CS, Dalamaga M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism 2019;92: 121-135.
14. Colditz GA, Peterson LL. Obesity and Cancer: Evidence, Impact, and Future Directions. Clin Chem 2018;64: 154-162.
15. Ho JSY, Fernando DI, Chan MY, Sia CH. Obesity in COVID-19: A Systematic Review and Meta-analysis. Ann Acad Med Singap 2020;49: 996-1008.
16. Poly TN, Islam MM, Yang HC, Lin MC, Jian WS, Hsu MH, et al. Obesity and Mortality Among Patients Diagnosed With COVID-19: A Systematic Review and Meta-Analysis. Front Med (Lausanne) 2021;8: 620044.
17. Sales-Peres SHC, de Azevedo-Silva LJ, Bonato RCS, Sales-Peres MC, Pinto A, Santiago Junior JF. Coronavirus (SARS-CoV-2) and the risk of obesity for critically illness and ICU admitted: Meta-analysis of the epidemiological evidence. Obes Res Clin Pract 2020;14: 389-397.
18. Huang Y, Lu Y, Huang YM, Wang M, Ling W, Sui Y, et al. Obesity in patients with COVID-19: a systematic review and meta-analysis. Metabolism 2020;113: 154378.
19. NCD-Risk-Factor-Collaboration. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet (London, England) 2016;387: 1377-1396.
20. NCD-Risk-Factor-Collaboration. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet (London, England) 2017;390: 2627-2642.
21. Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N Engl J Med 2017;377: 13-27.
22. NCD-Risk-Factor-Collaboration. Height and body-mass index trajectories of school-aged children and adolescents from 1985 to 2019 in 200 countries and territories: a pooled analysis of 2181 population-based studies with 65 million participants. Lancet (London, England) 2020;396: 1511-1524.
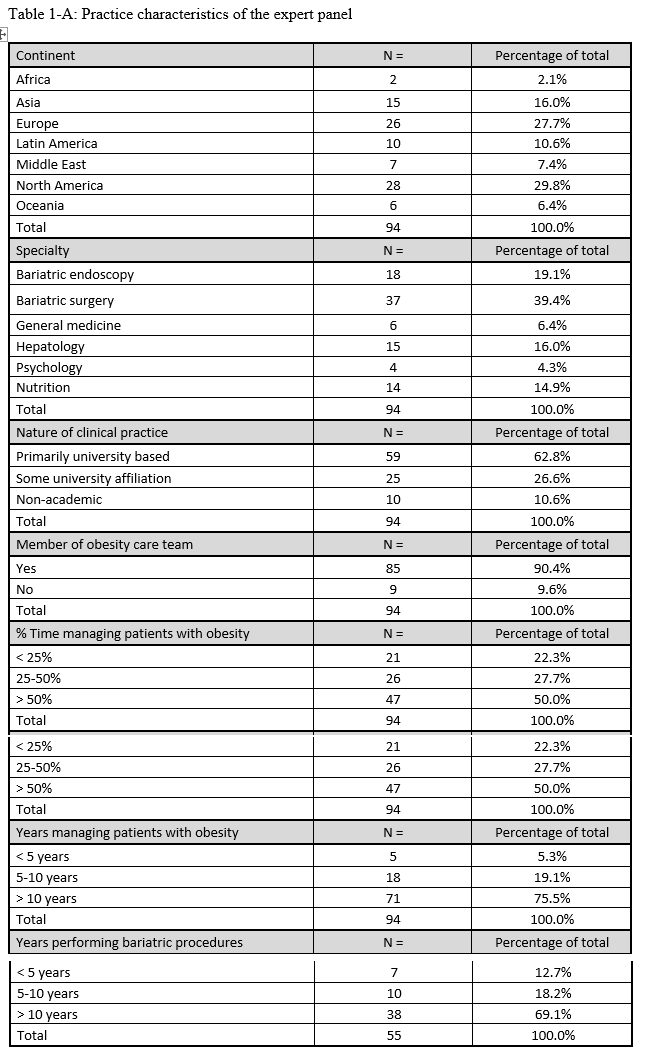
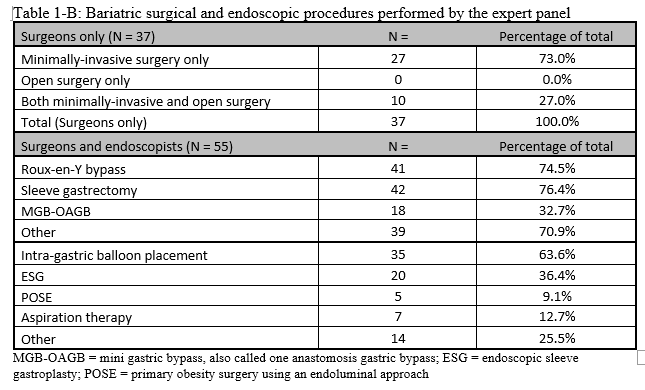
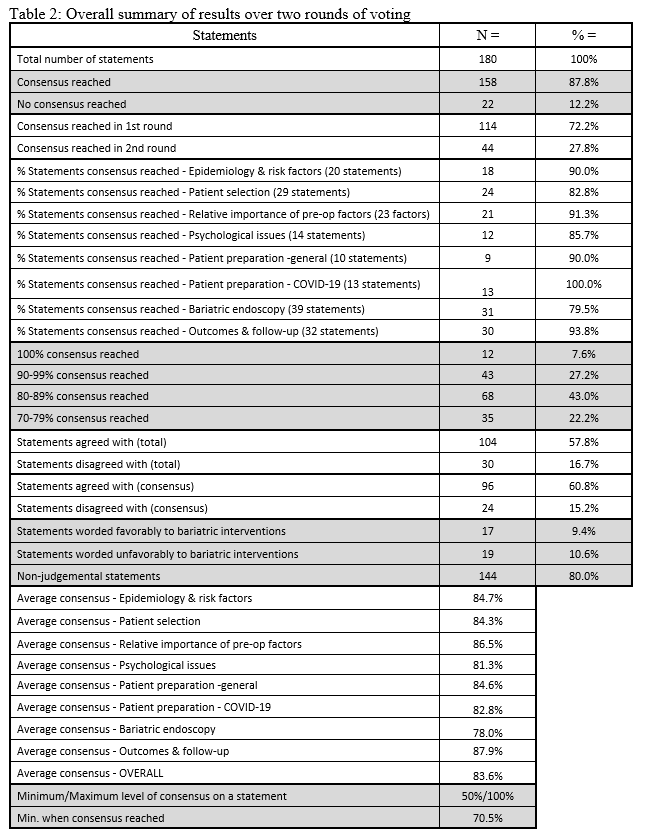
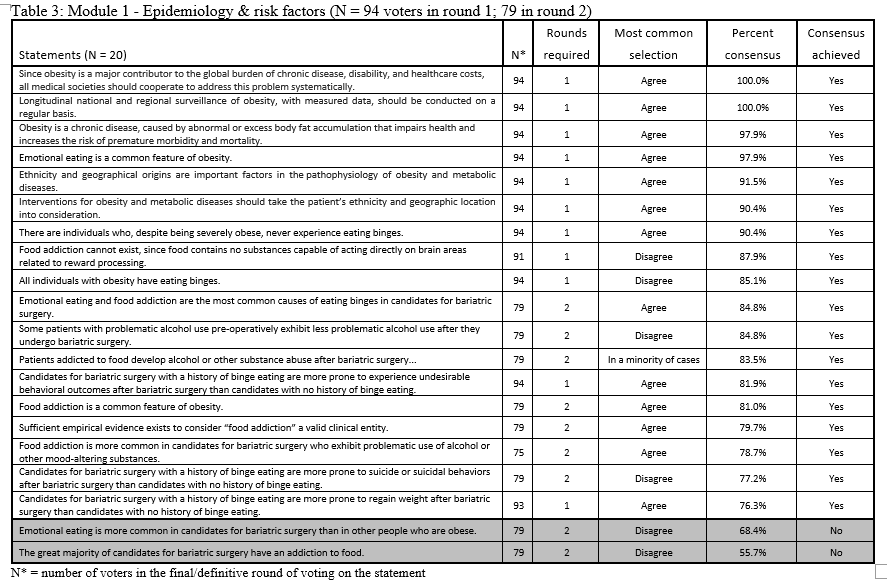
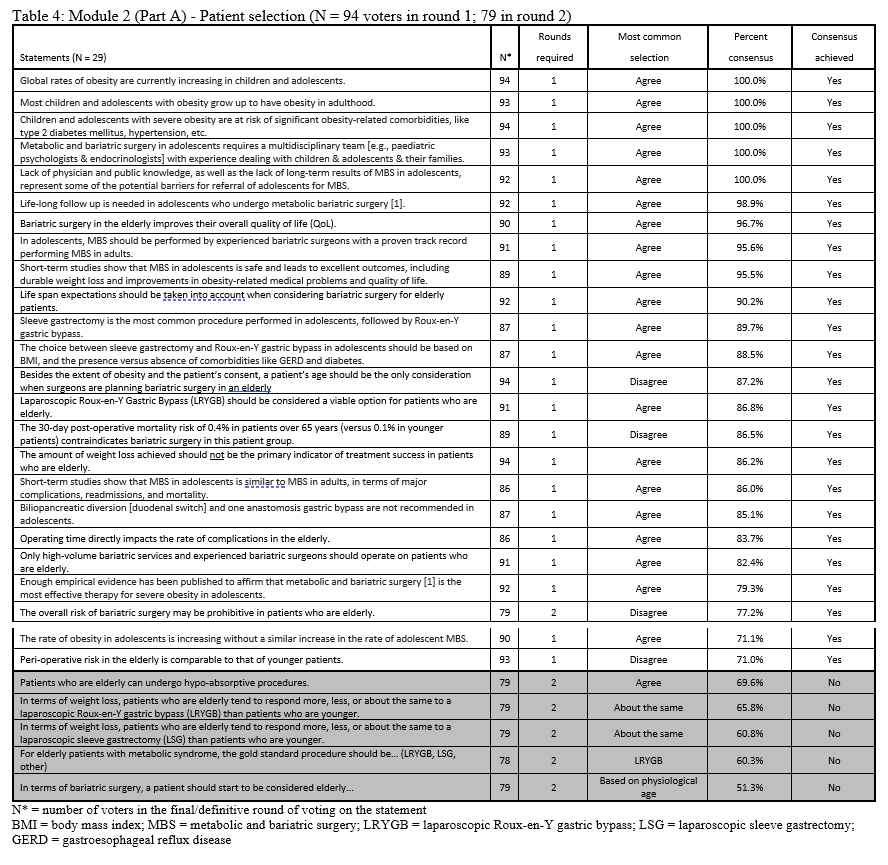
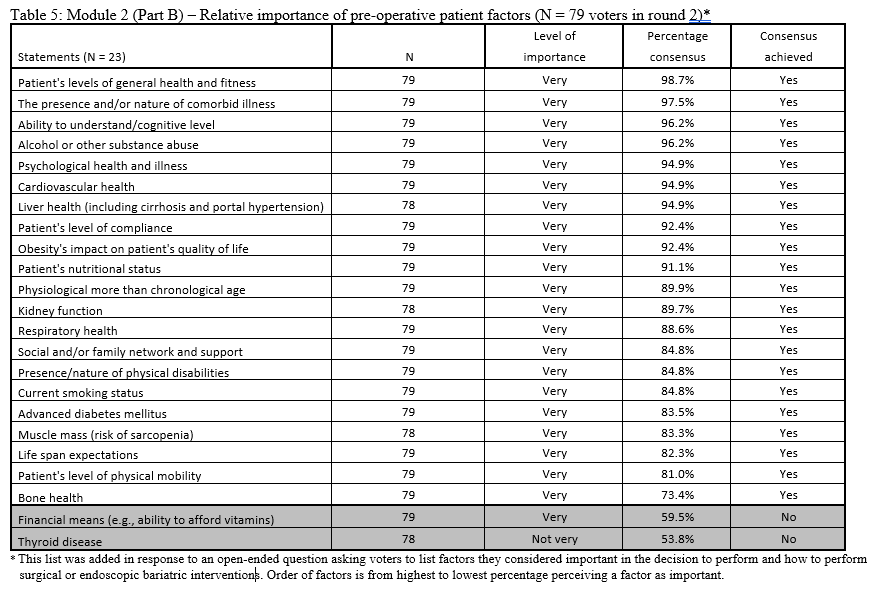
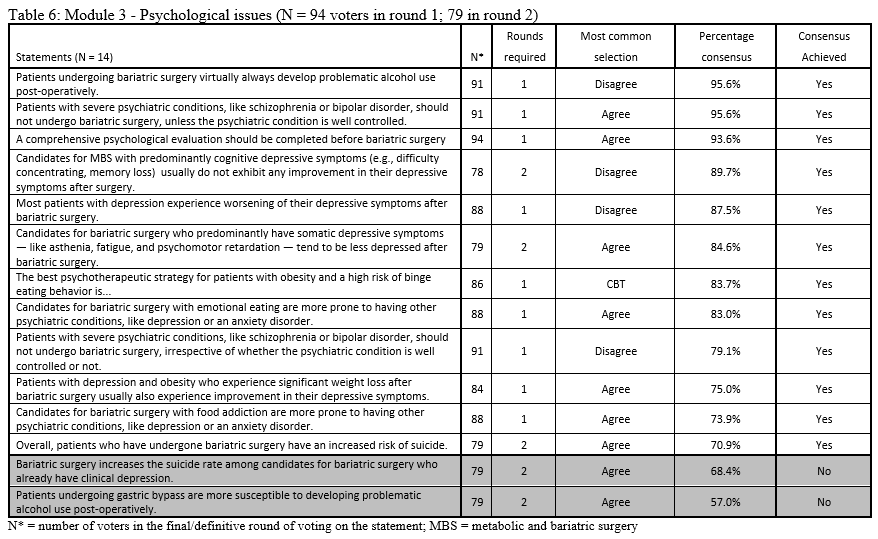
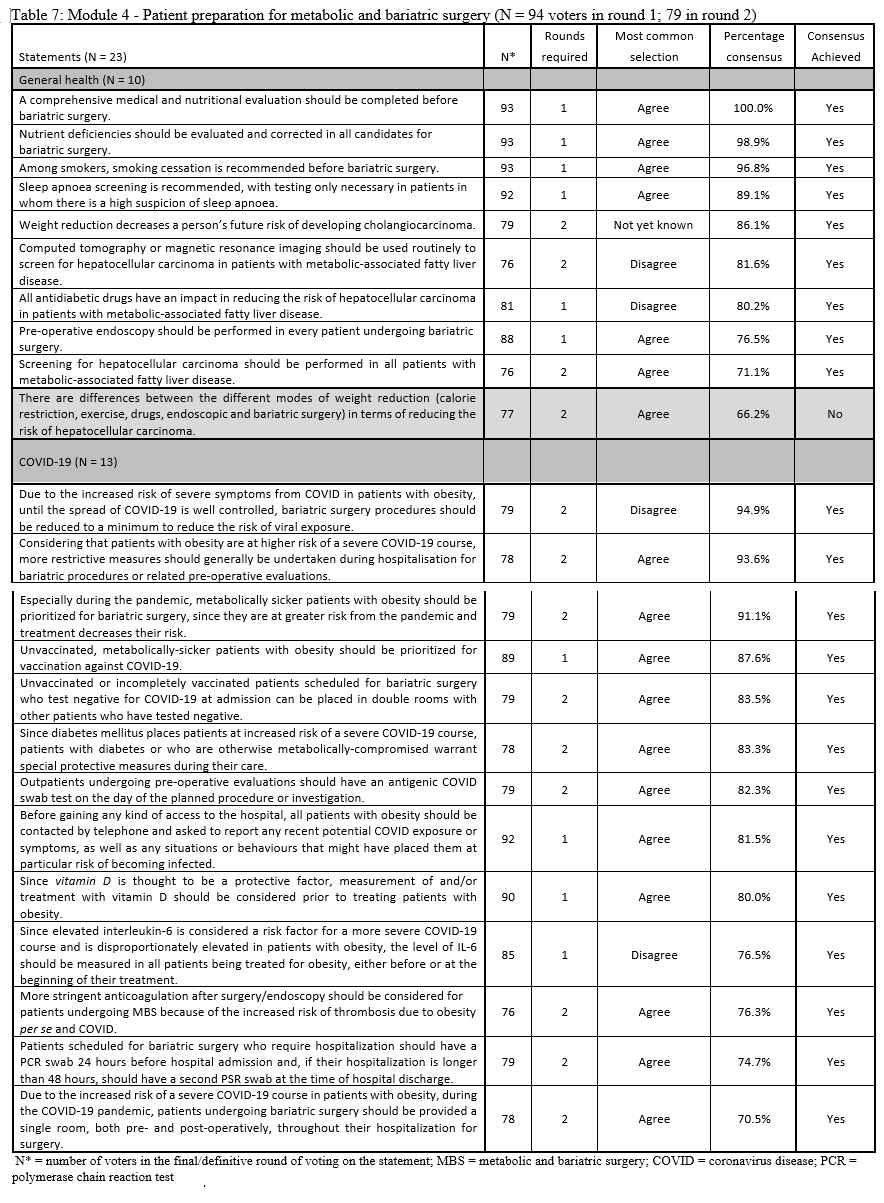
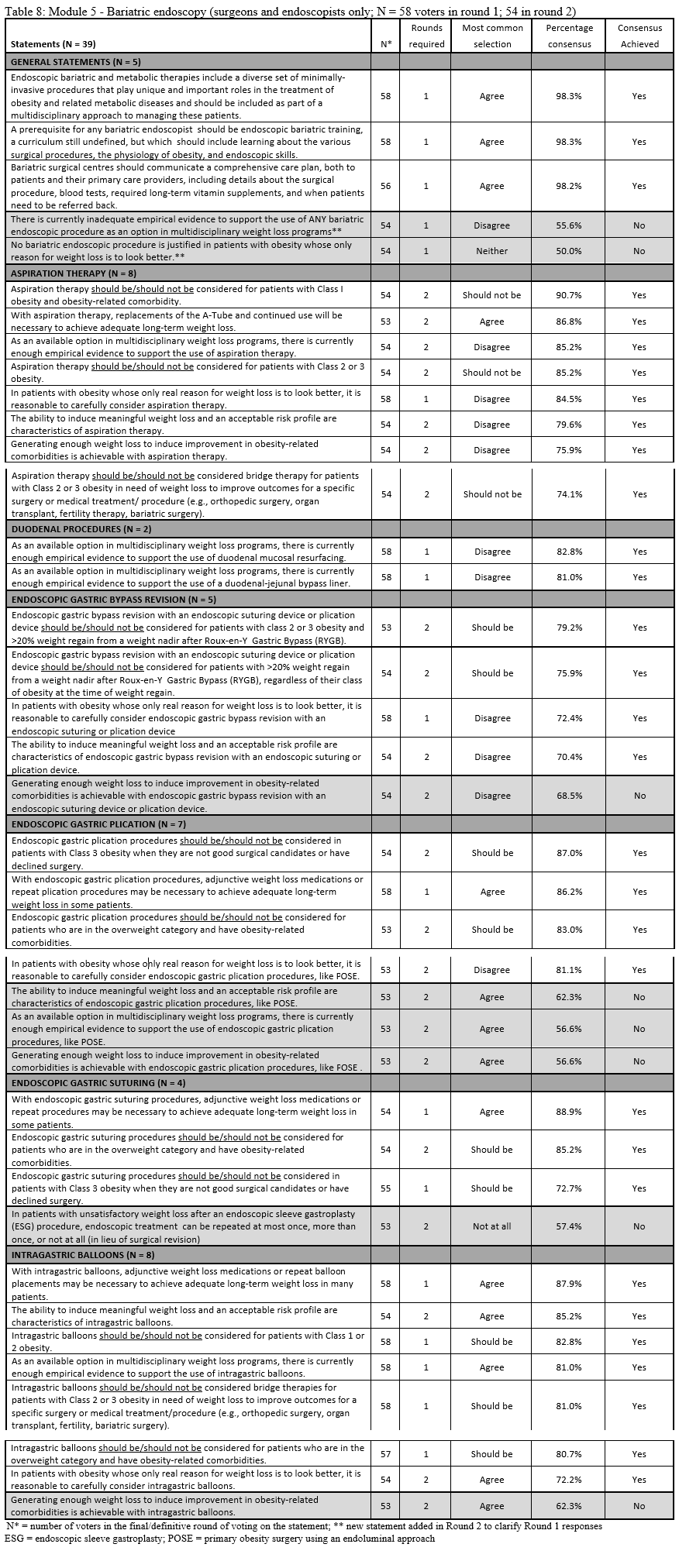
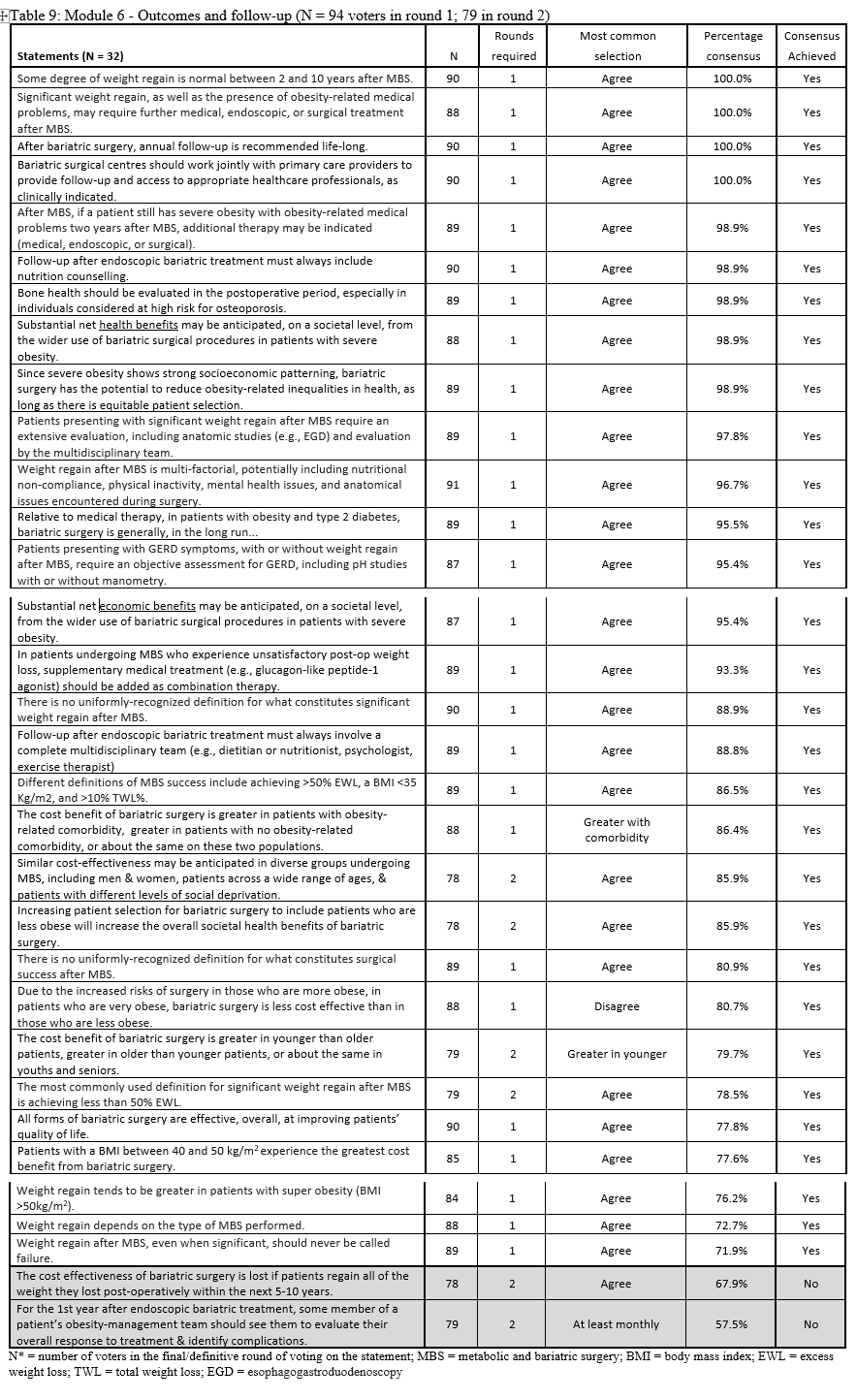
23. Lilian Kow, Mary O’Kane, Kevin P. White, Guilherme Macedo, Reem Shahaira, Jim Toouli, et al. Methodology and results of a joint IFSO-WGO Delphi Survey of 94 intercontinental, interdisciplinary experts in obesity management. [publication pending] 2023.
24. Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature 2006;444: 881-887.
25. Vega GL. Obesity and the metabolic syndrome. Minerva Endocrinol 2004;29: 47-54.
26. Scheen AJ, Luyckx FH. Obesity and liver disease. Best Pract Res Clin Endocrinol Metab 2002;16: 703-716.
27. Schuppan D, Schattenberg JM. Non-alcoholic steatohepatitis: pathogenesis and novel therapeutic approaches. J Gastroenterol Hepatol 2013;28 Suppl 1: 68-76.
28. Smith BW, Adams LA. Non-alcoholic fatty liver disease. Crit Rev Clin Lab Sci 2011;48: 97-113.
29. Bonfrate L, Wang DQ, Garruti G, Portincasa P. Obesity and the risk and prognosis of gallstone disease and pancreatitis. Best Pract Res Clin Gastroenterol 2014;28: 623-635.
30. Cruz-Monserrate Z, Conwell DL, Krishna SG. The Impact of Obesity on Gallstone Disease, Acute Pancreatitis, and Pancreatic Cancer. Gastroenterol Clin North Am 2016;45: 625-637.
31. Nimeri AA, Gamaleldin MM, McKenna KL, Turrin NP, Mustafa BO. Reduction of Venous Thromboembolism in Surgical Patients Using a Mandatory Risk-Scoring System: 5-Year Follow-Up of an American College of Surgeons National Surgical Quality Improvement Program. Clin Appl Thromb Hemost 2017;23: 392-396.
32. Rodrigues AFS, Korkes F, Bezerra DSD, Freitas Júnior WR, Toledo LGM. Impact of bariatric surgery in patients with stress urinary incontinence. Einstein (Sao Paulo) 2021;19: eAO5701.
33. Sheridan W, Da Silva AS, Leca BM, Ostarijas E, Patel AG, Aylwin SJ, et al. Weight loss with bariatric surgery or behaviour modification and the impact on female obesity-related urine incontinence: A comprehensive systematic review and meta-analysis. Clinical obesity 2021;11: e12450.
34. Kalyvas A, Neromyliotis E, Koutsarnakis C, Komaitis S, Drosos E, Skandalakis GP, et al. A systematic review of surgical treatments of idiopathic intracranial hypertension (IIH). Neurosurg Rev 2021;44: 773-792.
35. Mollan SP, Aguiar M, Evison F, Frew E, Sinclair AJ. The expanding burden of idiopathic intracranial hypertension. Eye (Lond) 2019;33: 478-485.
36. Blagojevic M, Jinks C, Jeffery A, Jordan KP. Risk factors for onset of osteoarthritis of the knee in older adults: a systematic review and meta-analysis. Osteoarthritis Cartilage 2010;18: 24-33.
37. Gadalla TM. Association of obesity with mood and anxiety disorders in the adult general population. Chronic Dis Can 2009;30: 29-36.
38. Kolotkin RL, Meter K, Williams GR. Quality of life and obesity. Obes Rev 2001;2: 219-229.
39. Kushner RF, Foster GD. Obesity and quality of life. Nutrition 2000;16: 947-952.
40. Mannucci E, Petroni ML, Villanova N, Rotella CM, Apolone G, Marchesini G. Clinical and psychological correlates of health-related quality of life in obese patients. Health Qual Life Outcomes 2010;8: 90.
41. Riazi A, Shakoor S, Dundas I, Eiser C, McKenzie SA. Health-related quality of life in a clinical sample of obese children and adolescents. Health Qual Life Outcomes 2010;8: 134.
42. Angrisani L, Santonicola A, Iovino P, Vitiello A, Zundel N, Buchwald H, et al. Bariatric Surgery and Endoluminal Procedures: IFSO Worldwide Survey 2014. Obesity surgery 2017;27: 2279-2289.
43. Malik VS, Willett WC, Hu FB. Global obesity: trends, risk factors and policy implications. Nat Rev Endocrinol 2013;9: 13-27.
44. Seidell JC, Halberstadt J. The global burden of obesity and the challenges of prevention. Ann Nutr Metab 2015;66 Suppl 2: 7-12.
45. Reynolds CL, Byrne SM, Hamdorf JM. Treatment Success: Investigating Clinically Significant Change in Quality of Life Following Bariatric Surgery. Obesity surgery 2017;27: 1842-1848.
46. Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol 2014;63: 2985-3023.
47. Mechanick JI, Apovian C, Brethauer S, Garvey WT, Joffe AM, Kim J, et al. CLINICAL PRACTICE GUIDELINES FOR THE PERIOPERATIVE NUTRITION, METABOLIC, AND NONSURGICAL SUPPORT OF PATIENTS UNDERGOING BARIATRIC PROCEDURES - 2019 UPDATE: COSPONSORED BY AMERICAN ASSOCIATION OF CLINICAL ENDOCRINOLOGISTS/AMERICAN COLLEGE OF ENDOCRINOLOGY, THE OBESITY SOCIETY, AMERICAN SOCIETY FOR METABOLIC & BARIATRIC SURGERY, OBESITY MEDICINE ASSOCIATION, AND AMERICAN SOCIETY OF ANESTHESIOLOGISTS - EXECUTIVE SUMMARY. Endocr Pract 2019;25: 1346-1359.
48. National-Institute-for-Health-and-Care-Excellence. NICE CG189 Obesity: identification, assessment and management of overweight and obesity in children, young people and adults. London: National Institute for Health and Care Excellence; 2014 [Internet] 2021 [cited 2021 30 April 2021]. Available from: http://www.nice.org.uk/guidance/cg189.
49. Mechanick JI, Apovian C, Brethauer S, Timothy Garvey W, Joffe AM, Kim J, et al. Clinical Practice Guidelines for the Perioperative Nutrition, Metabolic, and Nonsurgical Support of Patients Undergoing Bariatric Procedures - 2019 Update: Cosponsored by American Association of Clinical Endocrinologists/American College of Endocrinology, The Obesity Society, American Society for Metabolic and Bariatric Surgery, Obesity Medicine Association, and American Society of Anesthesiologists. Obesity (Silver Spring) 2020;28: O1-o58.
50. Aarts MA, Sivapalan N, Nikzad SE, Serodio K, Sockalingam S, Conn LG. Optimizing Bariatric Surgery Multidisciplinary Follow-up: a Focus on Patient-Centered Care. Obesity surgery 2017;27: 730-736.
51. Fletcher PC, Kenny PJ. Food addiction: a valid concept? Neuropsychopharmacology 2018;43: 2506-2513.
52. Heinberg LJ, Ashton K, Coughlin J. Alcohol and bariatric surgery: review and suggested recommendations for assessment and management. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery 2012;8: 357-363.
53. Bordignon S, Aparício MJG, Bertoletti J, Trentini CM. Personality characteristics and bariatric surgery outcomes: a systematic review. Trends Psychiatry Psychother 2017;39: 124-134.
54. Kirk S, Ramos Salas X, Alberga AS, S. R-M. Canadian Adult Obesity Clinical Practice Guidelines: Reducing Weight Bias, Stigma and Discrimination in Obesity Management, Practice and Policy 2021 [cited 2021 30 April 2021]. Available from: https://obesitycanada.ca/guidelines/weightbias/.
55. Wharton S, Lau DCW, Vallis M, Sharma AM, Biertho L, Campbell-Scherer D, et al. Obesity in adults: a clinical practice guideline. Cmaj 2020;192: E875-e891.
56. Puhl RM, Heuer CA. The stigma of obesity: a review and update. Obesity (Silver Spring) 2009;17: 941-964.
57. Teachman BA, Brownell KD. Implicit anti-fat bias among health professionals: is anyone immune? Int J Obes Relat Metab Disord 2001;25: 1525-1531.
58. Vallis MT, Currie B, Lawlor D, Ransom T. Healthcare Professional Bias Against the Obese: How Do We Know If We Have a Problem? Canadian Journal of Diabetes 2007;31: 365-370.
59. Parrott J, Frank L, Rabena R, Craggs-Dino L, Isom KA, Greiman L. American Society for Metabolic and Bariatric Surgery Integrated Health Nutritional Guidelines for the Surgical Weight Loss Patient 2016 Update: Micronutrients. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery 2017;13: 727-741.
60. Poitou Bernert C, Ciangura C, Coupaye M, Czernichow S, Bouillot JL, Basdevant A. Nutritional deficiency after gastric bypass: diagnosis, prevention and treatment. Diabetes Metab 2007;33: 13-24.
61. Stroh C, Manger T, Benedix F. Metabolic surgery and nutritional deficiencies. Minerva chirurgica 2017;72: 432-441.
62. Ziegler O, Sirveaux MA, Brunaud L, Reibel N, Quilliot D. Medical follow up after bariatric surgery: nutritional and drug issues. General recommendations for the prevention and treatment of nutritional deficiencies. Diabetes Metab 2009;35: 544-557.
63. O'Kane M, Parretti HM, Pinkney J, Welbourn R, Hughes CA, Mok J, et al. British Obesity and Metabolic Surgery Society Guidelines on perioperative and postoperative biochemical monitoring and micronutrient replacement for patients undergoing bariatric surgery-2020 update. Obes Rev 2020;21: e13087.
64. Parrott JM, Craggs-Dino L, Faria SL, O'Kane M. The Optimal Nutritional Programme for Bariatric and Metabolic Surgery. Curr Obes Rep 2020;9: 326-338.
65. Quilliot D, Coupaye M, Ciangura C, Czernichow S, Sallé A, Gaborit B, et al. Recommendations for nutritional care after bariatric surgery: Recommendations for best practice and SOFFCO-MM/AFERO/SFNCM/expert consensus. J Visc Surg 2021;158: 51-61.
66. Sherf-Dagan S, Sinai T, Goldenshluger A, Globus I, Kessler Y, Schweiger C, et al. Nutritional Assessment and Preparation for Adult Bariatric Surgery Candidates: Clinical Practice. Adv Nutr 2021;12: 1020-1031.
67. Hassannejad A, Khalaj A, Mansournia MA, Rajabian Tabesh M, Alizadeh Z. The Effect of Aerobic or Aerobic-Strength Exercise on Body Composition and Functional Capacity in Patients with BMI ≥35 after Bariatric Surgery: a Randomized Control Trial. Obesity surgery 2017;27: 2792-2801.
68. Jakicic JM, Davis KK. Obesity and physical activity. Psychiatr Clin North Am 2011;34: 829-840.
69. Neumann CR, Marcon ER, CG M. Princípios, Formação e Prática. In: Gusso G, JMC L (eds). Tratado de Medicina de Família e Comunidade Art. Med., 2012, pp 1417-1427.
70. Lau DC, Douketis JD, Morrison KM, Hramiak IM, Sharma AM, Ur E. 2006 Canadian clinical practice guidelines on the management and prevention of obesity in adults and children [summary]. Cmaj 2007;176: S1-13.
71. Parikh M, Liu J, Vieira D, Tzimas D, Horwitz D, Antony A, et al. Preoperative Endoscopy Prior to Bariatric Surgery: a Systematic Review and Meta-Analysis of the Literature. Obesity surgery 2016;26: 2961-2966.
72. Bello-Chavolla OY, Bahena-López JP, Antonio-Villa NE, Vargas-Vázquez A, González-Díaz A, Márquez-Salinas A, et al. Predicting Mortality Due to SARS-CoV-2: A Mechanistic Score Relating Obesity and Diabetes to COVID-19 Outcomes in Mexico. J Clin Endocrinol Metab 2020;105.
73. Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. Bmj 2020;369: m1985.
74. Petrilli CM, Jones SA, Yang J, Rajagopalan H, O'Donnell L, Chernyak Y, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. Bmj 2020;369: m1966.
75. Sattar N, Ho FK, Gill JM, Ghouri N, Gray SR, Celis-Morales CA, et al. BMI and future risk for COVID-19 infection and death across sex, age and ethnicity: Preliminary findings from UK biobank. Diabetes & metabolic syndrome 2020;14: 1149-1151.
76. Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020;584: 430-436.
77. Goel R, Nasta AM, Goel M, Prasad A, Jammu G, Fobi M, et al. Complications after bariatric surgery: A multicentric study of 11,568 patients from Indian bariatric surgery outcomes reporting group. Journal of minimal access surgery 2021;17: 213-220.
78. Pories WJ. Bariatric surgery: risks and rewards. J Clin Endocrinol Metab 2008;93: S89-96.
79. Marczuk P, Kubisa MJ, Święch M, Walędziak M, Kowalewski P, Major P, et al. Effectiveness and Safety of Roux-en-Y Gastric Bypass in Elderly Patients-Systematic Review and Meta-analysis. Obesity surgery 2019;29: 361-368.
80. Giordano S, Victorzon M. Bariatric surgery in elderly patients: a systematic review. Clin Interv Aging 2015;10: 1627-1635.
81. Volkert D, Beck AM, Cederholm T, Cruz-Jentoft A, Goisser S, Hooper L, et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin Nutr 2019;38: 10-47.
82. Chow A, Switzer NJ, Gill RS, Dang J, Ko YM, Shi X, et al. Roux-en-Y Gastric Bypass in the Elderly: a Systematic Review. Obesity surgery 2016;26: 626-630.
83. Domienik-Karłowicz J, Pruszczyk P, Lisik W. Bariatric Surgery in the Elderly Patient: Safety and Short-Time Outcome. A Case Match Analysis: Letter to the Editor. Obesity surgery 2019;29: 1658.
84. Gray KD, Moore MD, Bellorin O, Abelson JS, Dakin G, Zarnegar R, et al. Increased Metabolic Benefit for Obese, Elderly Patients Undergoing Roux-en-Y Gastric Bypass vs Sleeve Gastrectomy. Obesity surgery 2018;28: 636-642.
85. Shenoy SS, Gilliam A, Mehanna A, Kanakala V, Bussa G, Gill T, et al. Laparoscopic Sleeve Gastrectomy Versus Laparoscopic Roux-en-Y Gastric Bypass in Elderly Bariatric Patients: Safety and Efficacy-a Systematic Review and Meta-analysis. Obesity surgery 2020;30: 4467-4473.
86. Kaplan U, Penner S, Farrokhyar F, Andruszkiewicz N, Breau R, Gmora S, et al. Bariatric Surgery in the Elderly Is Associated with Similar Surgical Risks and Significant Long-Term Health Benefits. Obesity surgery 2018;28: 2165-2170.
87. Balasundaram P, Krishna S. Obesity Effects On Child Health. StatPearls. StatPearls Publishing
Copyright © 2022, StatPearls Publishing LLC.: Treasure Island (FL), 2022.
88. Gurnani M, Birken C, Hamilton J. Childhood Obesity: Causes, Consequences, and Management. Pediatr Clin North Am 2015;62: 821-840.
89. Sahoo K, Sahoo B, Choudhury AK, Sofi NY, Kumar R, Bhadoria AS. Childhood obesity: causes and consequences. J Family Med Prim Care 2015;4: 187-192.
90. Gordon-Larsen P, Adair LS, Nelson MC, Popkin BM. Five-year obesity incidence in the transition period between adolescence and adulthood: the National Longitudinal Study of Adolescent Health. Am J Clin Nutr 2004;80: 569-575.
91. Fontaine KR, Redden DT, Wang C, Westfall AO, Allison DB. Years of life lost due to obesity. Jama 2003;289: 187-193.
92. Poliakin L, Roberts A, Thompson KJ, Raheem E, McKillop IH, Nimeri A. Outcomes of adolescents compared with young adults after bariatric surgery: an analysis of 227,671 patients using the MBSAQIP data registry. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery 2020;16: 1463-1473.
93. ASMBS-Clinical-Issues-Committee. Peri-operative management of obstructive sleep apnea. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery 2012;8: e27-32.
94. Pinhas-Hamiel O, Hamiel U, Bendor CD, Bardugo A, Twig G, Cukierman-Yaffe T. The Global Spread of Severe Obesity in Toddlers, Children, and Adolescents: A Systematic Review and Meta-Analysis. Obesity facts 2022;15: 118-134.
95. Malhotra S, Czepiel KS, Akam EY, Shaw AY, Sivasubramanian R, Seetharaman S, et al. Bariatric surgery in the treatment of adolescent obesity: current perspectives in the United States. Expert Rev Endocrinol Metab 2021;16: 123-134.
96. Jirapinyo P, Thompson CC. Endoscopic Bariatric and Metabolic Therapies: Surgical Analogues and Mechanisms of Action. Clin Gastroenterol Hepatol 2017;15: 619-630.
97. Sullivan S, Edmundowicz SA, Thompson CC. Endoscopic Bariatric and Metabolic Therapies: New and Emerging Technologies. Gastroenterology 2017;152: 1791-1801.
98. Alsabah S, Al Haddad E, Ekrouf S, Almulla A, Al-Subaie S, Al Kendari M. The safety and efficacy of the procedureless intragastric balloon. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery 2018;14: 311-317.
99. Bazerbachi F, Haffar S, Sawas T, Vargas EJ, Kaur RJ, Wang Z, et al. Fluid-Filled Versus Gas-Filled Intragastric Balloons as Obesity Interventions: a Network Meta-analysis of Randomized Trials. Obesity surgery 2018;28: 2617-2625.
100. Ienca R, Al Jarallah M, Caballero A, Giardiello C, Rosa M, Kolmer S, et al. The Procedureless Elipse Gastric Balloon Program: Multicenter Experience in 1770 Consecutive Patients. Obesity surgery 2020;30: 3354-3362.
101. Jamal MH, Almutairi R, Elabd R, AlSabah SK, Alqattan H, Altaweel T. The Safety and Efficacy of Procedureless Gastric Balloon: a Study Examining the Effect of Elipse Intragastric Balloon Safety, Short and Medium Term Effects on Weight Loss with 1-Year Follow-Up Post-removal. Obesity surgery 2019;29: 1236-1241.
102. Kumar N, Bazerbachi F, Rustagi T, McCarty TR, Thompson CC, Galvao Neto MP, et al. The Influence of the Orbera Intragastric Balloon Filling Volumes on Weight Loss, Tolerability, and Adverse Events: a Systematic Review and Meta-Analysis. Obesity surgery 2017;27: 2272-2278.
103. Stavrou G, Shrewsbury A, Kotzampassi K. Six intragastric balloons: Which to choose? World J Gastrointest Endosc 2021;13: 238-259.
104. Trang J, Lee SS, Miller A, Cruz Pico CX, Postoev A, Ibikunle I, et al. Incidence of nausea and vomiting after intragastric balloon placement in bariatric patients - A systematic review and meta-analysis. International journal of surgery (London, England) 2018;57: 22-29.
105. Hedjoudje A, Abu Dayyeh BK, Cheskin LJ, Adam A, Neto MG, Badurdeen D, et al. Efficacy and Safety of Endoscopic Sleeve Gastroplasty: A Systematic Review and Meta-Analysis. Clin Gastroenterol Hepatol 2020;18: 1043-1053.e1044.
106. Jalal MA, Cheng Q, Edye MB. Systematic Review and Meta-Analysis of Endoscopic Sleeve Gastroplasty with Comparison to Laparoscopic Sleeve Gastrectomy. Obesity surgery 2020;30: 2754-2762.
107. Marincola G, Gallo C, Hassan C, Raffaelli M, Costamagna G, Bove V, et al. Laparoscopic sleeve gastrectomy versus endoscopic sleeve gastroplasty: a systematic review and meta-analysis. Endosc Int Open 2021;9: E87-e95.
108. Yoon JY, Arau RT. The Efficacy and Safety of Endoscopic Sleeve Gastroplasty as an Alternative to Laparoscopic Sleeve Gastrectomy. Clin Endosc 2021;54: 17-24.
109. Sharaiha RZ, Hajifathalian K, Kumar R, Saunders K, Mehta A, Ang B, et al. Five-Year Outcomes of Endoscopic Sleeve Gastroplasty for the Treatment of Obesity. Clin Gastroenterol Hepatol 2021;19: 1051-1057.e1052.
110. Carlsson LMS, Sjöholm K, Jacobson P, Andersson-Assarsson JC, Svensson PA, Taube M, et al. Life Expectancy after Bariatric Surgery in the Swedish Obese Subjects Study. N Engl J Med 2020;383: 1535-1543.
111. Rutledge R. The mini-gastric bypass: experience with the first 1,274 cases. Obesity surgery 2001;11: 276-280.
112. Mejía-Rivas MA, Herrera-López A, Hernández-Calleros J, Herrera MF, Valdovinos MA. Gastroesophageal reflux disease in morbid obesity: the effect of Roux-en-Y gastric bypass. Obesity surgery 2008;18: 1217-1224.
113. Qumseya BJ, Qumsiyeh Y, Ponniah SA, Estores D, Yang D, Johnson-Mann CN, et al. Barrett's esophagus after sleeve gastrectomy: a systematic review and meta-analysis. Gastrointest Endosc 2021;93: 343-352.e342.
114. Sebastianelli L, Benois M, Vanbiervliet G, Bailly L, Robert M, Turrin N, et al. Systematic Endoscopy 5 Years After Sleeve Gastrectomy Results in a High Rate of Barrett's Esophagus: Results of a Multicenter Study. Obesity surgery 2019;29: 1462-1469.
115. Stenard F, Iannelli A. Laparoscopic sleeve gastrectomy and gastroesophageal reflux. World journal of gastroenterology 2015;21: 10348-10357.
116. Morais M, Faria G, Preto J, Costa-Maia J. Gallstones and Bariatric Surgery: To Treat or Not to Treat? World journal of surgery 2016;40: 2904-2910.
117. Mulliri A, Menahem B, Alves A, Dupont B. Ursodeoxycholic acid for the prevention of gallstones and subsequent cholecystectomy after bariatric surgery: a meta-analysis of randomized controlled trials. J Gastroenterol 2022;57: 529-539.
118. Sneineh MA, Harel L, Elnasasra A, Razin H, Rotmensh A, Moscovici S, et al. Increased Incidence of Symptomatic Cholelithiasis After Bariatric Roux-En-Y Gastric Bypass and Previous Bariatric Surgery: a Single Center Experience. Obesity surgery 2020;30: 846-850.
119. Talha A, Abdelbaki T, Farouk A, Hasouna E, Azzam E, Shehata G. Cholelithiasis after bariatric surgery, incidence, and prophylaxis: randomized controlled trial. Surgical endoscopy 2020;34: 5331-5337.
120. Antozzi P, Soto F, Arias F, Carrodeguas L, Ropos T, Zundel N, et al. Development of acute gouty attack in the morbidly obese population after bariatric surgery. Obesity surgery 2005;15: 405-407.
121. Friedman JE, Dallal RM, Lord JL. Gouty attacks occur frequently in postoperative gastric bypass patients. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery 2008;4: 11-13.
122. Romero-Talamás H, Daigle CR, Aminian A, Corcelles R, Brethauer SA, Schauer PR. The effect of bariatric surgery on gout: a comparative study. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery 2014;10: 1161-1165.
123. Tana C, Busetto L, Di Vincenzo A, Ricci F, Ticinesi A, Lauretani F, et al. Management of hyperuricemia and gout in obese patients undergoing bariatric surgery. Postgrad Med 2018;130: 523-535.
124. Duffey BG, Pedro RN, Makhlouf A, Kriedberg C, Stessman M, Hinck B, et al. Roux-en-Y gastric bypass is associated with early increased risk factors for development of calcium oxalate nephrolithiasis. J Am Coll Surg 2008;206: 1145-1153.
125. Lieske JC, Mehta RA, Milliner DS, Rule AD, Bergstralh EJ, Sarr MG. Kidney stones are common after bariatric surgery. Kidney Int 2015;87: 839-845.
126. Thongprayoon C, Cheungpasitporn W, Vijayvargiya P, Anthanont P, Erickson SB. The risk of kidney stones following bariatric surgery: a systematic review and meta-analysis. Ren Fail 2016;38: 424-430.
127. Upala S, Jaruvongvanich V, Sanguankeo A. Risk of nephrolithiasis, hyperoxaluria, and calcium oxalate supersaturation increased after Roux-en-Y gastric bypass surgery: a systematic review and meta-analysis. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery 2016;12: 1513-1521.
128. Algahtani HA, Khan AS, Khan MA, Aldarmahi AA, Lodhi Y. Neurological complications of bariatric surgery. Neurosciences (Riyadh) 2016;21: 241-245.
129. Alligier M, Borel AL, Savey V, Rives-Lange C, Brindisi MC, Piguel X, et al. A series of severe neurologic complications after bariatric surgery in France: the NEUROBAR Study. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery 2020;16: 1429-1435.
130. Bal BS, Finelli FC, Shope TR, Koch TR. Nutritional deficiencies after bariatric surgery. Nat Rev Endocrinol 2012;8: 544-556.
131. Hadi YB, Mann R, Sohail AH, Shah-Khan SM, Szoka N, Abunnaja S, et al. Metabolic bone disease and fracture risk after gastric bypass and sleeve gastrectomy: comparative analysis of a multi-institutional research network. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery 2022;18: 604-609.
132. Krez AN, Stein EM. The Skeletal Consequences of Bariatric Surgery. Curr Osteoporos Rep 2020;18: 262-272.
133. Paccou J, Caiazzo R, Lespessailles E, Cortet B. Bariatric Surgery and Osteoporosis. Calcif Tissue Int 2022;110: 576-591.
134. Paccou J, Tsourdi E, Meier C, Palermo A, Pepe J, Body JJ, et al. Bariatric surgery and skeletal health: A narrative review and position statement for management by the European Calcified Tissue Society (ECTS). Bone 2022;154: 116236.
135. Ben-Porat T, Elazary R, Goldenshluger A, Sherf Dagan S, Mintz Y, Weiss R. Nutritional deficiencies four years after laparoscopic sleeve gastrectomy-are supplements required for a lifetime? Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery 2017;13: 1138-1144.
136. Madhok BM, Mahawar KK, Hadfield JN, Courtney M, Stubbing-Moore A, Koshy S, et al. Haematological indices and haematinic levels after mini gastric bypass: a matched comparison with Roux-en-Y gastric bypass. Clinical obesity 2018;8: 43-49.
137. Gombart AF, Pierre A, Maggini S. A Review of Micronutrients and the Immune System-Working in Harmony to Reduce the Risk of Infection. Nutrients 2020;12.
138. Mauro Lombardo, Arianna Franchi, Elvira Padua, Valeria Guglielmi, Monica D'Adamo, Giuseppe Annino, et al. Potential Nutritional Deficiencies in Obese Subjects 5 Years After Bariatric Surgery. Bariatric Surgical Practice and Patient Care 2019;14.
139. Nicoletti A, Ponziani FR, Biolato M, Valenza V, Marrone G, Sganga G, et al. Intestinal permeability in the pathogenesis of liver damage: From non-alcoholic fatty liver disease to liver transplantation. World journal of gastroenterology 2019;25: 4814-4834.
140. Shiau J, Biertho L. Canadian Adult Obesity Clinical Practice Guidelines: Bariatric Surgery: Postoperative Management. 2020. Available from: https://obesitycanada.ca/guidelines/postop/.
141. Trainer S, Benjamin T. Elective surgery to save my life: rethinking the "choice" in bariatric surgery. J Adv Nurs 2017;73: 894-904.
142. Vallis MT, Macklin D, Russell-Mayhew S. Canadian Adult Obesity Clinical Practice Guidelines: Effective Psychological and Behavioural Interventions in Obesity Management 2020. Available from: https://obesitycanada.ca/guidelines/behavioural/.
143. Cornejo-Pareja I, Molina-Vega M, Gómez-Pérez AM, Damas-Fuentes M, Tinahones FJ. Factors Related to Weight Loss Maintenance in the Medium-Long Term after Bariatric Surgery: A Review. J Clin Med 2021;10.
144. Eisenberg D, Noria S, Grover B, Goodpaster K, Rogers AM. ASMBS position statement on weight bias and stigma. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery 2019;15: 814-821.
145. Soricelli E, Casella G, Baglio G, Maselli R, Ernesti I, Genco A. Lack of correlation between gastroesophageal reflux disease symptoms and esophageal lesions after sleeve gastrectomy. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery 2018;14: 751-756.
146. Jirapinyo P, Thompson CC. Obesity Primer for the Practicing Gastroenterologist. Am J Gastroenterol 2021;116: 918-934.