1. Introduction
This is the second WGO guideline published to complement World Digestive Health Day (WDHD) themes. The WGO’s aim in the guideline is to guide health-care providers in the best management of gastroesophageal reflux disease (GERD) through a concise document that provides recommendations based on the latest evidence and has been drawn up in a global expert consensus process focusing on the best current practice.
1.1 Cascades for GERD diagnosis and management
WGO guidelines are intended to highlight appropriate, context-sensitive and resource- sensitive management options for all geographical regions, regardless of whether they are considered to be “developing,” “semi-developed,” or “developed.”
- There is a concern that guidelines from developed countries, by emphasizing high-tech investigations and Barrett’s esophagus (BE) surveillance, for example, may divert research and clinical resources from more urgent problems in developing and semideveloped countries.
- However, one could argue that there are similar problems in developed countries and that an overemphasis on complications or “proposed GERD associations” (as in the Montreal Consensus [1]) is leading to inappropriate investigations and resource utilization even in developed regions.
- It is also important to emphasize to health-care insurers and funding bodies that appropriate, effective therapy is both therapeutic and diagnostic and that conducting mandatory investigations (e.g., esophagogastroduodenoscopy) to permit proton-pump inhibitor (PPI) therapy is not patient-centered and, more importantly, is likely not to be cost-effective.
- WGO Cascades are thus context-sensitive, and the context is not necessarily defined solely by resource availability.
A standardized global approach would require that the epidemiology of GERD and reflux-like symptoms be comparable in all parts of the world, and that the full ranges of diagnostic tests and medical treatment options be generally available. However, neither the epidemiology of the condition, nor the availability of resources for the diagnosis and management of GERD, is sufficiently uniform throughout the world to support the provision of a single, gold standard approach.
GERD is a condition that is particularly suitable for the WGO cascade approach, and this global WGO guideline therefore includes a set of cascades to provide context-sensitive and resource-sensitive options for the diagnosis and management of GERD. The WGO Cascades are intended to serve as a “global” complement to — rather than a replacement for — the “gold standard” guidelines produced by regional groups and national societies.
• WGO Cascades: a hierarchical set of diagnostic, therapeutic, and management options for dealing with risk and disease, ranked by the resources available.
GERD is now widely prevalent around the world, with clear evidence of increasing prevalence in many developing countries. Practice recommendations should be sensitive to context, with the goal of optimizing care in relation to local resources and the availability of health-care support systems. The expression of the disease is considered to be similar across regions, with heartburn and regurgitation as the main symptoms. For initial management, the patient may purchase over-the-counter (OTC) medication for heartburn relief or seek further advice from a pharmacist. When patients perceive that their symptoms are more troublesome, they may seek a doctor’s advice; depending on the patient’s circumstances and the structure of the local health- care system, patients may seek advice at the primary care level or they may consult a gastroenterology specialist or surgeon, directly or by referral. The WGO cascade approach aims to optimize the use of available health-care resources for individual patients, based on their location and access to various health-care providers.
- In this guideline, the Cascades are listed in sections 3.5, “Cascades for the Diagnosis of GERD” and 4.6, “Cascades for the Management of GERD.”
- Section 5.2 in the Appendix provides a list of selected “gold standard” guidelines.
1.2 Definition and description of GERD
- Gastroesophageal reflux disease (GERD) can be defined as troublesome symptoms sufficient to impair an individual’s quality of life, or injury or complications that result from the retrograde flow of gastric contents into the esophagus, oropharynx, and/or respiratory tract.
- Reflux-induced symptoms, erosive esophagitis, and long-term complications [2] may have severely deleterious effects on daily activities, work productivity, sleep, and quality of life. The Montreal definition of GERD states that “troublesome symptoms” may be considered to be moderate to severe symptoms that occur on one or more days per week.
- GERD can be classified relative to the presence or absence of erosions; GERD symptoms without erosions on endoscopic examination constitute nonerosive reflux disease (NERD), whereas GERD symptoms with erosions constitute erosive esophagitis (EE) [3]. It should be emphasized that EE can also occur in the absence of symptoms [4].
- ERD has been defined specifically as “a subcategory of GERD characterized by troublesome reflux-related symptoms in the absence of esophageal mucosal erosions at conventional endoscopy and in the absence of recent acid-suppressive therapy.” This definition was further qualified [5] on the basis of pathobiology and diagnosis with the statement that “evidence in support of this diagnosis includes, but is not limited to, responsiveness to acid suppression, positive 24-h pH monitoring (positive symptom association) or identification of specific novel endoscopic, morphological, or physiological findings.” NERD is by far the most common form of GERD globally [5].
- Barrett’s esophagus (BE) refers to the endoscopic presence, confirmed histologically, of columnar-lined esophagus. This is currently the only identifiable complication of GERD that is known to have malignant potential.
- Extraesophageal GERD syndromes can be categorized as conditions that have an established association with GERD (cough, laryngitis, asthma, dental erosions) and those that have only a proposed association (pharyngitis, sinusitis, idiopathic pulmonary fibrosis, otitis media) [6].
Reflux-related symptoms occur with frequency and severity across a continuum. There are individuals who experience occasional, mild reflux symptoms that do not trouble them and are not considered to meet the criteria for a diagnosis of GERD; symptoms in such individuals should be managed with low-intensity, intermittent treatments and lifestyle adjustments as required.
- Reflux symptoms in NERD may be as severe as those experienced by patients with mucosal damage confirmed by endoscopy [7].
- The occurrence of reflux symptoms two or more times per week is associated with a reduction in the patient’s quality of life, even if the symptoms are mild [8]. Troublesome reflux symptoms have therefore been defined as those that occur two or more times per week [1].
- The occurrence of infrequent moderate or severe symptoms less than twice per week may nonetheless be sufficient to affect quality of life, consistent with a diagnosis of GERD [8].
1.3 Epidemiology of GERD
GERD is a global disease, and evidence suggests that its prevalence is increasing. Prevalence estimates show considerable geographic variation, but it is only in East Asia that prevalence estimates are currently consistently lower than 10% [9]. The high prevalence of GERD, and hence of troublesome symptoms, has significant societal consequences, impacting adversely on work productivity [10] and many other quality- of-life aspects for individual patients [11,12].
Robust epidemiological studies are still lacking for developed countries, such as Japan, as well as from many emerging economies including Russia, India, and the African continent. There are also few data regarding the prevalence of GERD in pediatric populations, the incidence of GERD [9] (Table 1), its natural history, and its causes.
Most epidemiological studies of the condition are symptom-based [4]. As symptom-based diagnosis is challenging, the epidemiological data on the prevalence of gastroesophageal reflux symptoms (GERS) are probably flawed. This is in part because the description and nomenclature of reflux symptoms varies between regions, and in part because upper gastrointestinal symptoms (“dyspeptic” symptoms) may be described similarly by patients who have a variety of upper gastrointestinal diagnoses, including peptic ulcer disease, nonulcer dyspepsia, dysmotility, or GERD [13]. Nonetheless, it is instructive to report the prevalence of “dyspeptic” symptoms across the globe, as these data affect the pretest probability that upper gastrointestinal symptoms are attributable to gastroesophageal reflux.
Table 1 GERD symptoms: range of incidence

2. Clinical features
2.1 Predisposing and risk factors
GERD is a sensorimotor disorder associated with impairment of the normal antireflux mechanisms (e.g., lower esophageal sphincter function, phrenicoesophageal ligament), with changes in normal physiology (e.g., impaired esophageal peristalsis, increased intragastric pressure, increased abdominothoracic pressure gradient) or, very rarely, excess gastric acid secretion (Zollinger–Ellison syndrome).
Eating and lifestyle
- An increase in GERD symptoms occurs in individuals who gain weight [14].
- A high body mass index (BMI) is associated with an increased risk of GERD [15].
- High dietary fat intake is linked to a higher risk of GERD and erosive esophagitis (EE) [16].
- Carbonated drinks are a risk factor for heartburn during sleep in patients with GERD [17].
- The role of coffee as a risk factor for GERD is unclear; coffee may increase heartburn in some GERD patients [18], but the mechanism is unknown and it may be due to caffeine, rather than coffee per se. Coffee is not a dominant risk factor.
- The role of alcohol consumption as a risk factor for GERD is unclear. Excessive, long-term use may be associated with progression to esophageal malignancy, but this may be independent of an effect of alcohol on GERD [19,20].
- The role of smoking as a risk factor for GERD is unclear, although like alcohol, it is associated with an increased risk of malignancy [21,22].
Medication — certain medications may affect GERD
See also section 3.2 on patient history and physical examination.
The treatment of comorbidities (e.g., with calcium channel blockers, anticholinergics, and nonsteroidal anti-inflammatory drugs (NSAIDs) may negatively affect GERD and its treatment [23]. Some medications (e.g., bisphosphonates, antibiotics, potassium supplements) may cause upper gastrointestinal tract injury and exacerbate reflux-like symptoms or reflux-induced injury.
Pregnancy
Heartburn during pregnancy usually does not differ from the classical presentation in the adult population, but it worsens as pregnancy advances. Regurgitation occurs with approximately the same frequency as heartburn, and GERD in the first trimester is associated with a number of altered physiological responses [24,25]. Factors that increase the risk of heartburn [26] are: heartburn before pregnancy, parity, and duration of pregnancy. Maternal age is inversely correlated with the occurrence of pregnancy-related heartburn [27].
Other pathobiological factors
- The higher incidence of GERD in Caucasians [28] is likely to be related to lifestyle rather than genetic factors.
- Comorbidities are frequent in patients with GERD: diabetes, metabolic syndrome, cardiovascular disease, and sleep apnea are all common. Overweight and obesity are common risk factors both for GERD and for these other comorbidities.
- GERD frequently coexists with other gastrointestinal syndromes such as irritable bowel syndrome.
- In Japan, osteoporosis with vertebral fractures and kyphosis is widely considered to be one of the risk factors for erosive esophagitis, especially among elderly women, and when severe, these skeletal conditions have been associated with Barrett’s epithelium [29–31].
2.2 Symptomatology
GERD has a wide spectrum of clinical symptom-based and injury-based presentations, which may manifest either separately or in combination.
- Heartburn and regurgitation are the cardinal and most common symptoms of GERD, but the definitions and relative prevalences of heartburn and regurgitation may vary regionally.
- Regurgitation may indicate gastroesophageal reflux, but it can occur with other less common conditions such as obstruction or achalasia.
- Regurgitation should be distinguished from rumination: rumination is the effortless regurgitation of partially digested food into the mouth, with either expulsion or further mastication and swallowing; rumination is behavioral.
- Heartburn is a retrosternal burning sensation that may move upward toward the neck and throat. It may coexist with other symptoms referable to the upper gastrointestinal tract; see the Montreal definition of GERD [1] (Table 2).
Table 2 The Montreal definition of GERD [1]

- Heartburn may be accompanied by regurgitation of sour/acid-tasting fluid or gastric contents into the mouth or the back of the throat — acid or food regurgitation. Regurgitation is defined somewhat differently in some regions or languages; for instance, in Japan, the definition of regurgitation often includes an acidic taste.
- The term “heartburn” has no equivalent in many languages — for example, Asian patients may perceive and describe heartburn as chest pain. “Wind” — usually meaning belching/eructation or distension and a desire to belch/eructate — is a predominant complaint of many patients with GERD as well as with other upper gastrointestinal “diseases” [6].
- In practice, there may be no clear differentiation between what are regarded as GERD symptoms and “dyspepsia” (broadly defined as symptoms felt in the upper abdomen). Indeed, the results of the Diamond study [32] question the value of heartburn and regurgitation as diagnostic symptoms of GERD [6].
- The Canadian Dyspepsia (CanDys) Working Group defined dyspepsia as “a symptom complex of epigastric pain or discomfort thought to originate in the upper gastrointestinal tract … that may include any of the following symptoms: heartburn, acid regurgitation, excessive burping/belching, increased abdominal bloating, nausea, feeling of abnormal or slow digestion, or early satiety” [13]. An endoscopic study in patients with uninvestigated dyspepsia revealed that esophageal findings (predominantly erosive esophagitis) were more commonly seen in patients whose reflux symptoms (heartburn and regurgitation) were most troublesome; however, the prevalences of gastric and duodenal findings were comparable in patients with reflux, ulcer, and dysmotility symptoms [33].
Symptom evaluation is key to the diagnosis of GERD, particularly in the evaluation of the effectiveness of therapy. Heartburn and regurgitation are the most common symptoms, but atypical symptoms of GERD may occur, with or without the common symptoms. Atypical symptoms may include epigastric pain [34] or chest pain [1,35], which may mimic ischemic cardiac pain, as well as cough and other respiratory symptoms that may mimic asthma or other respiratory or laryngeal disorders. Dysphagia may also occur. A minority of GERD patients have multiple unexplained symptoms, which may be associated with psychological distress [8].
Table 3 GERD symptoms [36,37]
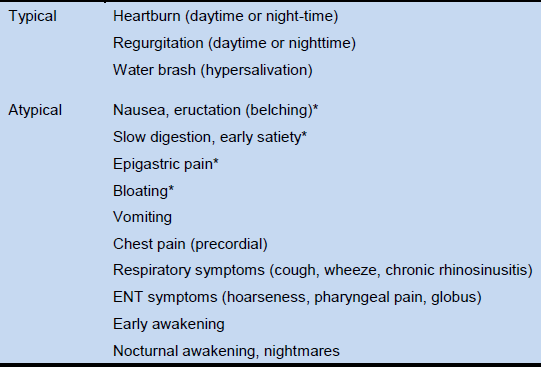
* Can be considered to be associated with GERD if symptoms improve in response to PPI treatment [38]. ENT, ear, nose and throat.
2.3 Natural history
- Most cases of GERD are mild and are not associated with a significant increase in morbidity or mortality in comparison with the general population.
- In most GERD patients, the severity of the condition remains stable or improves over a 5-year observation period during current routine clinical care [39].
- There is a relationship between GERD and obesity: a higher BMI or larger waist circumference and weight gain are associated with the presence of symptoms and complications of GERD, including BE [40].
- Complicated GERD is characterized by stricture, BE, and esophageal adenocarcinoma. The Montreal consensus includes erosive esophagitis (EE) as a complication of GERD (recognizing that the definition of “mucosal breaks” used in the Los Angeles classification includes esophageal ulceration in the range of reflux esophagitis) [41].
- NERD may progress to EE in approximately 10% of GERD patients [42], and EE may therefore be considered as a manifestation of more severe reflux disease.
- EE is associated with BE and is a major risk factor for BE. In comparison with patients who were free of GERD at follow-up, those with EE had a fivefold increased risk of BE after 5 years, in a cohort of the general population in Sweden [43].
- Globally, BE is rare in patients with GERD. It is more common in Western populations.
- It is not known when BE develops relative to the onset of GERD; however, it appears to be more prevalent in older individuals and is strongly associated with an increased risk of esophageal adenocarcinoma [44].
- There is a well-documented association between BMI and adenocarcinoma of the esophagus and gastric cardia, although the risk of malignancy in a given individual with GERD is very low [45].
2.4 Alarm features
Most alarm features are not specific for GERD; many are associated with alternative diagnoses that are unrelated to GERD. In most countries, many of these features relate to gastric cancer, complicated ulcer disease, or other serious illnesses.
- Dysphagia [46]
- Odynophagia (painful swallowing)
- Recurrent bronchial symptoms, aspiration pneumonia
- Dysphonia
- Recurrent or persistent cough
- Gastrointestinal tract bleeding
- Frequent nausea and/or vomiting
- Persistent pain
- Iron-deficiency anemia
- Progressive unintentional weight loss
- Lymphadenopathy
- Epigastric mass
- New-onset atypical symptoms at age 45–55 years. A lower age threshold may be appropriate, depending on local recommendations.
- Family history of either esophageal or gastric adenocarcinoma [6].
See also the WGO Global Guideline on common gastrointestinal symptoms (http://www.worldgastroenterology.org/guidelines/global-guidelines/common-gi- symptoms).
3. Diagnosis
3.1 Diagnostic considerations
The presence of heartburn and/or regurgitation symptoms two or more times a week is suggestive of GERD [47]. Clinical, endoscopic and pH-metric criteria provide a comprehensive characterization of the disease, although investigations are usually not required in order to establish a diagnosis of GERD — with the caveat that the pretest probability of GERD varies markedly between geographical regions. (See also section
1.2, Definition and description of GERD.)
The initial evaluation should document the presence, severity, and frequency of heartburn, regurgitation (acid or otherwise), and alarm features; atypical esophageal, pulmonary, otorhinolaryngological, and oral symptoms should also be sought. It may be helpful to evaluate precipitating factors such as eating, diet (fat), activity (stooping), and recumbence; and relieving factors (bicarbonate, antacids, milk, over- the counter medications) may be helpful.
At this point, it is important to rule out other gastrointestinal diagnoses, particularly upper gastrointestinal cancer and ulcer disease, especially in areas in which these are more prevalent. It is also important to consider other, nongastrointestinal diagnoses, especially ischemic heart disease.
Diagnostic questionnaire tools for GERD (reflux disease questionnaires, RDQs) have been developed for epidemiological studies. However, RDQs did not perform particularly well in the Diamond study [32]. In fact, diagnosis by a physician such as the family practitioner or gastrointestinal specialist showed better sensitivity and specificity for the diagnosis of GERD than did the RDQ. Questionnaires are generally difficult to use in clinical practice. A careful history is the basis for symptomatic diagnosis, with esophagogastroduodenoscopy (EGD) being reserved for identifying or excluding significant structural lesions in selected cases.
A region-based assessment of the local “pretest probability” may provide some guidance with regard to the choices and sequence of diagnostic tests needed, given the relatively poor predictive value of most symptoms.
PPI treatment as an aid to diagnosis
- “PPI trial.” It is no longer recommended to administer an empirical short-term (1–2-week) course of high-dose PPI treatment in order to determine whether or not the patient’s symptoms are acid-related [32], since this is neither sensitive nor specific. Nonetheless, this is commonly done in practice.
- A formal course of PPI therapy, of adequate duration (usually 8 weeks) is required in order to assess the treatment response in GERD patients.
- Weakly acidic reflux episodes may be a substantial proportion of all reflux episodes. If this is the case, such patients may not respond well to PPI therapy (20–40% of GERD patients may not respond to PPI treatment) [34]. In addition, genuinely alkaline reflux may comprise up to 5% of all reflux episodes.
- In a subset of PPI nonresponders, reflux-like symptoms may be due to functional heartburn, rather than GERD [34]. Alternative diagnoses, including peptic ulcer disease, upper gastrointestinal malignancy, functional dyspepsia, eosinophilic esophagitis, and achalasia of the cardia should also be considered.
- In patients with cases that are refractory to PPI treatment, ambulatory 24-hour esophageal pH/impedance monitoring, with the patient off PPI therapy, may be considered in order to help characterize symptoms [48].
- If there has been complete failure to respond to PPI treatment, the PPI should be stopped at least 1 week before 24-h pH monitoring is performed (rescue antacid may be allowed when necessary), in order to assess for acid reflux.
- If the refractory reflux symptoms have responded partially, 24-h pH monitoring (with or without esophageal impedance monitoring) should be performed with PPI administration being continued, in order to assess for acid reflux that is persistent despite treatment.
- Occasionally, 24-h pH monitoring, with esophageal impedance monitoring, may be required, both on and off PPI therapy [49].
Helicobacter pylori infection [50]
In many countries with a high prevalence of H. pylori, peptic ulcer and cancer continue to be more common than GERD and cause much greater morbidity and mortality [51].
- In this setting, an approach to the diagnosis and management of upper gut symptoms must integrate an assessment of the risks of H. pylori and an awareness of the overlap among and difficulty of discriminating between symptoms of GERD, peptic ulcer disease, and functional symptoms — with a decision regarding the relative merits of a test-and-treat approach in comparison with esophagogastroduodenoscopy (EGD) to test for H. pylori and related diseases before empirical antireflux therapy.
- Although epidemiological studies show a negative association between the prevalence of H. pylori infection and the presence and severity of GERD, this is not proof of causation. H. pylori infection should be sought and eradication therapy given when indicated in accordance with international, national, or local guidelines.
- The decline in the prevalence of H. pylori seen in some countries correlates with improving socioeconomic conditions. Improvements in levels of hygiene and sanitation reduce the likelihood of transmission of H. pylori (and other infectious diseases). Increasing socioeconomic status is closely associated with a rising prevalence of obesity, sedentary occupations, and altered dietary habits, all of which may promote reflux. Therefore, although there may be an inverse correlation between H. pylori and GERD prevalence and severity, this may well reflect differing effects of a separate, distinct factor or factors on the two conditions, rather than a causal relationship between H. pylori and GERD.
- Physiological studies using pH monitoring have shown that abnormal esophageal acid exposure, which is the hallmark of esophageal reflux, is not influenced by the presence or absence of H. pylori infection.
- In most patients, H. pylori status has no effect on symptom severity, symptom recurrence, or treatment efficacy in GERD. H. pylori eradication does not exacerbate preexisting GERD or affect treatment efficacy [52]. Indeed, in patients with H. pylori–positive uninvestigated dyspepsia, eradication therapy has been found to be associated with a lower prevalence of reflux-like symptoms (36%) than control therapy (49%) [53].
- A subgroup of patients infected with more proinflammatory strains of H. pylori (virulence factors vacA and cagA) may be less likely to have severe esophagitis or BE. This may be because infection in these patients more often causes severe corpus gastritis with atrophy, resulting in reduced acid output. However, these patients are at much greater risk of developing gastric cancer or ulcer. Eradication therapy in these patients has the potential to reduce the risk of gastric malignancy.
PPIs and H. pylori
The relationship between PPI therapy and the progression of gastritis and corpus atrophy in patients who have gastric H. pylori infection has been clarified since the initial observations by Kuipers et al. [54]. PPIs are associated with a worsening of the histological grade of gastritis in H. pylori–infected patients, accompanied by an increased prevalence of gastric mucosal atrophy and intestinal metaplasia [55] that occurs earlier, as well as more frequently, than in H. pylori–infected patients who do not take PPIs. This risk of gastric mucosal atrophy and intestinal metaplasia is not seen when PPIs are used in uninfected patients or in those who have had successful H. pylori eradication therapy before longer-term PPI use. As gastric mucosal atrophy and intestinal metaplasia are known to be the major risk factors for the development of gastric adenocarcinoma, most expert guidelines recommend testing and treating for H. pylori before long-term PPI therapy is administered, particularly in younger patients.
Endoscopy (EGD)
EGD is usually performed for new-onset upper gastrointestinal symptoms, almost irrespective of age, in regions where it is available and affordable and where both the frequency of ulcer disease and the concern about malignancy are high, as in most of Asia [56]. The Cascades given below address the limited availability of endoscopy in less well-resourced areas by suggesting the use of empirical H. pylori eradication therapy as a first-line strategy.
- If EGD is performed in regions where the prevalence of GERD is low, the majority of GERD patients will have NERD; in these circumstances, the sensitivity of EGD for the diagnosis of GERD will be low and the main outcome will therefore be the exclusion of other upper gastrointestinal diagnoses.
- Endoscopy is particularly recommended for patients with alarm features suggestive of GERD with complications or of other significant upper gastrointestinal disease such as dysphagia, bleeding, odynophagia, or weight loss.
- Patients with dysphagia should undergo investigation for a potential complication or for an underlying motility disorder, achalasia, stricture, ring, eosinophilic esophagitis, or malignancy [38].
- In several Asian countries, the preference for EGD is driven by the risk of malignancy at an early age and by the availability of “affordable, direct access” endoscopy — an “endoscopy first” approach.
Other investigations
Additional investigations other than EGD are rarely needed; furthermore, they have variable accuracy and are often unavailable.
- Additional relevant investigations include radiological examination, scintigraphy, manometry, and prolonged esophageal pH monitoring, with or without esophageal impedance monitoring.
- Esophageal pH or impedance pH monitoring for 24 hours (or 48–72 hours with the Bravo esophageal pH capsule) may be used to quantify esophageal acid exposure and to evaluate the temporal association between heartburn and reflux episodes, using a measure such as the symptom-association probability [57].
- Esophagus investigations are usually ordered or performed by a specialist, after consultation; they are rarely required except for specific patients with recalcitrant or atypical symptoms. Even in the developed world, access to pH monitoring, impedance monitoring, manometry, and scintigraphy is often very limited.
3.2 Patient history and physical examination
The goals of patient evaluation include the assessment of symptoms and risk factors for the diagnosis of GERD and the prediction of long-term sequelae. In this regard, it is important to consider the regional epidemiology of upper gastrointestinal disease and the pretest probability of GERD relative to other conditions. For instance, in Asia, BE is uncommon, and it is not therefore an important risk for esophageal adenocarcinoma, which is itself uncommon. The prevalence of peptic ulcer and gastric cancer are the greater drivers of endoscopy in Asia where, unlike in the West, esophageal adenocarcinoma is less common. The increasing prevalence in the West of gastroesophageal junction cancers is probably related to GERD as well, even though these cancers are still uncommon. Conversely, squamous cancer is more common in other parts of the world (with a higher prevalence in Iran, for example), related to factors other than reflux. Consideration of all these factors together should guide the sequence and choice of diagnostic investigations.
Personal and family history features
The following features may be helpful in making a diagnosis and assessing the severity of GERD:
- Predisposing factors and risk factors (see above), including family history.
- Duration of symptoms.
- Daytime symptoms, including time of day and relationship to meals.
- Nocturnal symptoms, including impact on sleep and the effects of a recumbent position and large, late evening meals.
- Treatments and remedies tried, including symptomatic response to therapy; symptom improvement with acid-lowering medications including antacids supports a diagnosis of GERD.
- Periodic dysphagia or food bolus impaction may suggest reflux-related esophageal injury, stricture or malignancy, as well as eosinophilic esophagitis or esophageal dysmotility [58].
Drug history — inquire about medications that may contribute to upper gut symptoms (not necessarily GERD)
- Aspirin/nonsteroidal anti-inflammatory drugs (NSAIDs), iron, potassium, quinidine, tetracycline, bisphosphonates
- Zidovudine, anticholinergic agents, alpha-adrenergic antagonists, barbiturates
- β2-adrenergic agonists, calcium channel blockers, benzodiazepines, dopamine
- Estrogens, narcotic analgesics, nitrates, progesterone, prostaglandins, theophylline
- Tricyclic antidepressants, chemotherapy
Dietary history
- In some patients, bloating or constipation may be associated with an increased risk of GERD or GERS [59].
- Several studies suggest that stopping smoking and some physical measures, as well as modification of meal size and timing, can be beneficial, but there is limited evidence for the avoidance of alcohol and certain dietary ingredients including carbonated drinks, caffeine, fat, spicy foods, chocolate and mint [60].
- In those who are overweight, weight loss may be associated with improvement in GERD or GERS [61].
- Fermentable carbohydrates may increase the propensity for reflux [62].
Physical evaluation — there are usually no physical signs of GERD
- Waist circumference, weight, and BMI are relevant to risk.
- Peripheral stigmata of scleroderma may, rarely, be present.
- Evaluation and inspection to exclude other medical problems such as asthma, cardiac disease, and cancer:
- Anemia, weight loss
- Oropharynx: ulcerations, candidiasis, lesions, masses, lingual dental erosions, caries
- Neck: nodes, masses
- Lungs: wheezes, crackles
- Ears: hearing loss, middle ear effusions (evidence does not support gastroesophageal reflux as a cause of otitis media)
- Abdomen: masses, tenderness
- Signs (local or systemic) of malignancy if history and examination are suspicious
3.3 Diagnostic tests for GERD
A presumptive diagnosis of GERD can be established in the setting of typical symptoms: heartburn and regurgitation. In pregnancy, GERD can be reliably diagnosed on the basis of symptoms alone.
If the dominant or most troublesome symptoms are atypical for GERD, other diagnoses should be considered, including H. pylori–related diseases and NSAID- induced symptoms. In regions with a high prevalence of H. pylori infection, an initial H. pylori test-and-treat strategy, or endoscopy if available, should be considered.
Radiological examinations are seldom required. Esophageal pH or pH-impedance monitoring and esophageal manometry can be performed safely, but are seldom required. Intractable reflux symptoms or GERD complications can be evaluated safely using EGD [24,25].
- Upper gastrointestinal endoscopy (EGD) is not required in the presence of typical GERD symptoms, despite the high specificity of a finding of esophageal erosions or mucosal breaks [41] for a diagnosis of GERD. Endoscopy is recommended in the presence of alarm symptoms and for assessment of patients who are at higher risk for complications or other diagnoses [41]. The endoscopic features of reflux disease have been defined by the Genval, Montreal, and Vevey consensus groups and in the Los Angeles classification of GERD [1,5,41,63]. Recent data indicate that it is reasonable to perform endoscopy to screen for BE in certain high-risk groups [64] — in particular, overweight white males over the age of 50 with chronic GERD symptoms have an increased risk of esophageal adenocarcinoma.
- Endoscopic biopsies may be taken from the esophagus, stomach, or duodenum. Distal esophageal biopsies are not recommended for diagnosing GERD [65] and should not be taken, unless one is evaluating for complications [66] or eosinophilic esophagitis. If eosinophilic esophagitis is suspected on the basis of the patient’s history or endoscopic findings, biopsies should be taken from the distal and mid-esophagus [58]. In addition, four-quadrant esophageal biopsies should be taken if the endoscopic appearances are consistent with BE, with an endoscopic suspicion of esophageal metaplasia (ESEM) [34], or if there are visible abnormalities consistent with malignancy or infection. Gastric biopsies should be taken in order to diagnose H. pylori infection, atrophy, intestinal metaplasia, or dysplasia, even in the presence of erosive esophagitis. It should be recognized that biopsies may be false-negative for H. pylori if the patients are taking or have recently taken PPIs or antibiotics. There is no role for routine duodenal biopsies in patients with typical GERD symptoms.
- Urea breath testing (UBT; 13C or 14C) or H. pylori stool antigen testing are recommended as noninvasive tests for active H. pylori infection, as a basis for a “test-and-treat” strategy for H. pylori in regions in which the prevalence of H. pylori exceeds 20% [50]. H. pylori testing does not confirm or exclude a diagnosis of GERD, but in line with the Cascades approach, the diagnosis of upper gastrointestinal symptoms should be guided by local disease prevalence and economic factors.
- Serology is suboptimal for the diagnosis of active H. pylori infection, but in regions of high prevalence, particularly if the patient has not taken antibiotics recently, serology will still have a reasonably high positive predictive value, if locally validated. H. pylori serology may provide guidance in patients taking PPI therapy, which can lead to false-negative tests of active infection (UBT, H. pylori stool antigen test, histology, culture or rapid urease test).
- Esophageal manometry is recommended for preoperative evaluation, before anti- reflux surgery or for patients with persistent symptoms, despite adequate treatment and a normal endoscopy, to rule out achalasia or other motility disorders [3]. Esophageal manometry has no role in the routine diagnosis of GERD.
- Ambulatory esophageal pH-metry and impedance may help evaluate patients who are refractory to PPI therapy, and when the diagnosis of GERD is in question. Ambulatory reflux monitoring with pH-metry is the only test that can assess reflux symptom association [48]. Esophageal pH impedance monitoring may be helpful in patients with persistent reflux-like symptoms who have responded poorly to standard therapy [34], to assess both acid and non-acid reflux disease, but symptom association measures have not been validated for pH impedance monitoring. Esophageal pH monitoring is indicated before considering antireflux surgery for GERD, usually with the patients off therapy, in order to confirm that the symptoms are indeed reflux-related.
- Barium radiography (swallow or meal) should not be performed to diagnose GERD [67]. Barium radiography may be appropriate in patients who have ancillary symptoms of dysphagia, in order to assess structural disorders (e.g., hiatal hernia, malrotation) or motility disorders (e.g., achalasia).
Table 4 Diagnostic options for GERD
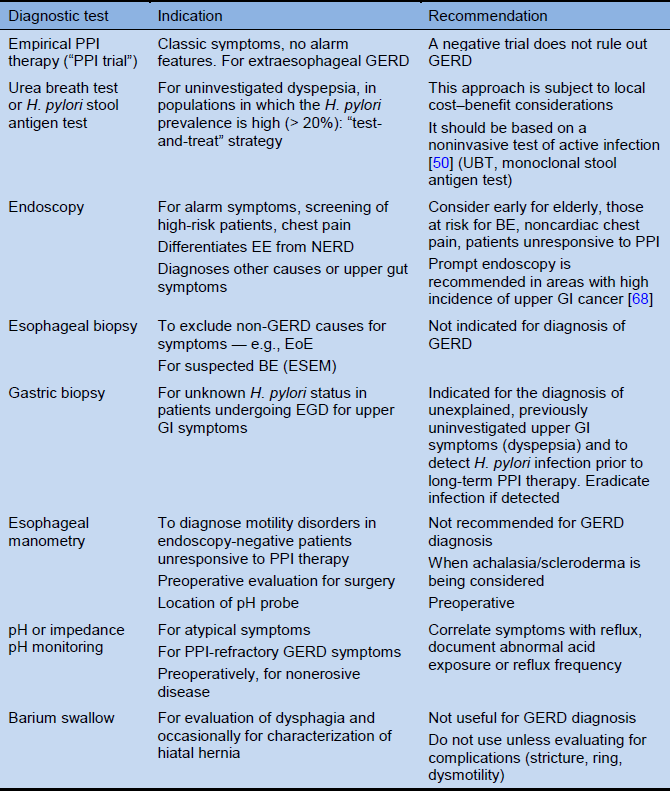
Based on: Katz et al. [3]. BE, Barrett’s esophagus; EE, erosive esophagitis; EGD, esophagogastroduodenoscopy; EoE, eosinophilic esophagitis; ESEM, endoscopic suspicion of esophageal metaplasia; GERD, gastroesophageal reflux disease; GI, gastrointestinal; NERD, nonerosive reflux disease; PPI, proton-pump inhibitors; UBT, urea breath test.
Note: The definition of NERD is based on investigations, and it is probably not relevant to the diagnosis and management of GERD by family practitioners and other community-based health-care providers, such as pharmacists.
3.4 Differential diagnosis
- Peptic ulcer disease
- Upper gut malignancy
- Functional heartburn — differentiate NERD and functional heartburn on the basis of a clinical response to therapeutic acid suppression, pH monitoring, or impedance pH monitoring
- Schatzki ring, stricture — esophageal web
- Achalasia of the cardia
- Esophageal body motility disorders — scleroderma; diffuse esophageal spasm
- Eosinophilic esophagitis
- Infection — Candida, herpes simplex, etc.
- “Pill esophagitis”
- Cardiac disease — ischemic heart disease, pericardial disease
- Esophageal diverticulum
- Other chest pathology
3.5 Cascades for the diagnosis of GERD
Table 5 Cascades for the diagnosis of GERD

BE, Barrett’s esophagus; b.i.d., bis in die (twice a day); EGD, esophagogastroduodenoscopy; o.d., omni die (daily); PPI, proton-pump inhibitor.
Notes:
* Alarm features warrant EGD in all regions.
** H. pylori prevalence:
Low: < 30% nationally, low-risk population, confirmed eradication.
High: ≥ 30% nationally, older patients, high-risk region (e.g., First Nations in North America), high-risk ethnic groups (immigrants from eastern Europe, South America, Africa, Indian subcontinent, Asia).
- For EGD, perform esophageal biopsy in abundantly resourced regions or biopsy for selected patients in regions with “medium resources” if features suggest eosinophilic esophagitis.
- For screening EGD, consider this only if there is a high prevalence of BE in the local population and if there are abundant resources.
- For most purposes, EGD will not alter the management, in the absence of alarm features or access to antireflux surgery.
- There is no role for upper gastrointestinal series in the investigation of routine upper gastrointestinal symptoms (uninvestigated dyspepsia).
4. Management
4.1 Management principles
General principles
While the severity and frequency of symptoms vary greatly between GERD patients, occasional reflux symptoms (GERS) do not meet the criteria for a diagnosis of GERD and are managed with low-level intermittent treatments and lifestyle adjustments, as required. More frequent or severe symptoms interfere significantly with patients’ quality of life and warrant therapy sufficient to normalize their quality of life.
Generally, the management of GERD follows a stepwise approach, both with respect to the therapies and to the health-care professionals who guide or provide therapy.
Core principles
The core principles of GERD management are lifestyle interventions, reduction of esophageal luminal acid either by local acid neutralization or by suppression of gastric acid secretion using medical treatment; or, rarely, antireflux surgery. The primary goals of treatment are to relieve symptoms, improve the patient’s health-related quality of life, heal esophagitis, prevent symptom recurrence, and prevent or treat GERD-associated complications in the most cost-effective manner.
4.2 Stepwise therapy
Infrequent heartburn occurring less than twice per week will probably respond to self- care with an antacid or alginate–antacid, taken once a week or less often. These medications are very unlikely to have any deleterious effects. Alginate–antacid combinations are useful and are superior to antacids alone [69]. Particularly in this group of patients, avoidance of foods or events that trigger symptoms and avoidance of large meals eaten late at night may be helpful. Weight reduction in those who are overweight may also reduce the frequency of symptoms.
Patients who have more frequent symptoms should be assessed for longer-term therapy. A diagnosis of GERD — i.e., troublesome symptoms two or more times per week — warrants empirical therapy with an acid inhibitor (PPI or, if unavailable, H2RA). Antacids/alginates may also be used if PPIs or H2RAs are unavailable, or for prompt symptom relief in patients taking acid suppressive medications.
If over-the-counter or lifestyle measures fail, patients will often present in the first instance to a pharmacist or primary care physician. The definition of treatment failure depends to a large extent on the treatment being tried. On the one hand, treatment may fail because the patient does not actually have GERD; on the other hand, it may be that the treatment is inadequate to address the severity of the GERD. In the latter case, there may be a partial response to therapy, and the subsequent management will be guided by the availability and optimization of more potent therapies. These latter steps may require referral to secondary care if initial management fails [70]. Approaches to reflux should focus on best clinical practice, with treatment of the symptoms being the priority.
- It is wise to choose the lowest effective dose of prescription drugs — the lowest dose capable of providing acceptable symptom relief. This may range from no drug to short-term treatment with a once-daily PPI. In practice, standard-dose PPI therapy is usually used first; half-dose PPI controls symptoms in fewer patients, although some patients can successfully “step down” to lower doses after initial symptom control on standard doses.
- For patients with mild symptoms, and some patients with endoscopically diagnosed NERD, self-directed, intermittent PPI therapy (“on-demand therapy”) is a useful management strategy in many cases. It reduces the number of tablets taken, reduces costs, and empowers patients to manage their own symptoms. However, reversion to daily therapy should occur if symptom control is poor and quality of life remains impaired.
- At the primary care level, PPIs or a combination of alginate–antacid and acid- suppressive therapy can be prescribed at the physician’s discretion for combination therapy, which may be more beneficial than acid-suppressive therapy alone [70].
- For better symptom control, patients should be informed about how to use PPI therapy properly; optimal therapy may be defined as taking the PPI 30–60 min before breakfast, and in the case of twice daily dosing, 30–60 min before the last meal of the day as well [71].
- Patients in whom full-dose PPI treatment fails, with or without adjuvant therapies, may benefit from a trial of step-up therapy to a twice-daily PPI.
- Twice-daily PPI therapy may not work for a proportion of patients, either because the symptoms are not due to acid reflux and an alternative diagnosis should be considered, or because the degree of acid suppression achieved is insufficient to control the symptoms. Referral to secondary care should be considered for these “PPI-refractory” patients.
- OTC antacids show disappointing results in patients with erosive esophagitis.
Self-care
- Controlled weight reduction in the overweight and obese is an important part of the long-term management of GERD and should not be ignored as a therapeutic intervention, as it may reduce the frequency and intensity of symptoms and lessen the grade of EE, if present.
- Lifestyle — small meals, avoidance of late meals, avoidance of precipitating factors, use of a sleep positioning device (pillow) [72].
- OTC medicines (antacids or alginate–antacids) offer the most rapid, but usually transient, symptom relief and can be taken as required.
- Alarm features: see section 2.4.
Options for pharmacist-assisted self-medication
- Reinforce lifestyle advice.
- Guide patients in the selection of medical OTC treatment by confirming the diagnosis, referring patients with alarm symptoms to physicians, and educating patients on the proper use of their OTC medication — which in some jurisdictions may include PPIs [73].
N.B.: the availability of treatment choices varies between countries.
- Antacids — recommended for short-term or intermittent relief:
- Simple antacids neutralize gastric acid — i.e., sodium, calcium, magnesium, and aluminum salts.
- Alginate-containing agents: these include alginic acid with small doses of antacids: minimal buffering effects.
- Histamine H2-receptor antagonists (H2RAs) — recommended for short-term to medium-term use
- Widely available OTC
- Cimetidine, ranitidine, famotidine, nizatidine
- More prolonged action than antacids
- Tachyphylaxis
- Over-the-counter PPIs:
- Patients seeking pharmacy advice for frequent reflux symptoms may benefit from OTC PPI treatment
- Esomeprazole, lansoprazole, omeprazole, pantoprazole, rabeprazole, which have different OTC availability in individual countries — see the Association of the European Self-Medication Industry web site (http://www.aesgp.eu/facts-figures/otc-ingredients/)
- Other OTC PPIs may be available in other jurisdictions.
- Alarm features: see section 2.4.
- Check medication interactions.
Self-treatment without investigation should be avoided in the presence of the following conditions [74–77]:
- Heartburn or regurgitation symptoms when:
- Duration > 3 months with severe or nocturnal heartburn
- Continuing after 2 weeks of treatment with an OTC H2RA or PPI
- Occurring when taking a prescription H2RA or PPI
- New-onset heartburn or regurgitation at age 45–55 years — lower age in several Asian regions
- Dysphagia or odynophagia
- Symptoms or signs of gastrointestinal bleeding: hematemesis and melena, iron- deficiency anemia
- Symptoms or signs of laryngitis: hoarseness, wheezing, coughing, or choking
- Unexplained weight loss
- Continuous nausea, vomiting, and/or diarrhea
- Symptoms suggestive of cardiac-type chest pain: radiating to shoulder, arm, neck or jaw, shortness of breath, sweating
- In pregnant women or nursing mothers
- Children < 12 years of age for antacids/H2RA, or < 18 years for PPIs.
Follow-up action
- The goals of self-treatment are that the patient should become symptom-free and return to an optimal quality of life, with the most cost-effective therapy.
- If satisfactory and complete symptom relief is not achieved, patients should be recommended to visit a health-care professional for diagnostic evaluation.
- PPI overuse — people who need sustained gastric acid suppression should have an appropriate indication for long-term PPI use; the long-term need for PPIs should be reassessed regularly. We advocate responsible PPI prescription, which should be based on good investigation and diagnosis and if the treatment does not work, medication should be stopped. Proper documentation is advocated.
Options for family physicians
- Reinforce lifestyle modifications
- Endorse OTC medications (antacids and alginates, H2RAs) as appropriate
- Prescription H2RAs
- Currently available PPIs — daily standard doses from studies of healing in EE (not all PPIs may be available in all countries, and the standard dose of PPIs may differ in some countries):
- Omeprazole (20 mg)
- Rabeprazole (20 mg)
- Lansoprazole (30 mg)
- Pantoprazole (40 mg)
- Esomeprazole (40 mg)
- Dexlansoprazole (60 mg)
- Prokinetic drugs:
- May decrease gastroesophageal reflux, but few prokinetics are available for clinical use and their efficacy in clinical trials has been modest at best. Not recommended.
- Metoclopramide should be avoided, because of adverse effects.
- Domperidone shows little benefit and is not recommended, because of safety concerns around prolongation of the QTc interval on electrocardiography.
- Mosapride: limited availability and efficacy.
- Alarm features: see section 2.4.
- Check medication interactions
- Rule out / treat other contributing conditions (constipation, exacerbating medications).
Options for specialists (secondary care: gastroenterologist, surgeon)
To address patients’ needs, the full range of symptoms should be taken into account. Symptoms in addition to or other than heartburn may respond differently to treatment.
- Regurgitation may not respond to treatment as well as heartburn.
- Interrupting PPI treatment may lead to short-term symptom rebound in a minority of patients [78,79].
- PPI treatment failure [80,81] may be related to:
- Incorrect diagnosis: common with functional heartburn.
- Noncompliance: patients with GERD may show poor adherence to the prescribed PPI, and this may play an important role in treatment failure [82].
- Incorrect dosing time: most PPIs are more effective if taken 30–60 min before a meal.
- Inadequate dosing.
- Low drug bioavailability (rapid metabolizers).
- Duodenogastroesophageal reflux, nocturnal reflux, weakly acidic reflux, residual acid reflux.
- Delayed/prolonged gastric emptying, gastric outlet obstruction.
- Esophageal hypersensitivity.
- Eosinophilic esophagitis.
- Psychological comorbidity.
- H2RAs are effective for suppressing acid in the short-term, but tachyphylaxis limits long-term benefits.
- There is little evidence to support the use of prokinetics (cisapride, domperidone, tegaserod, mosapride) alone or in combination with acid suppression. Serious adverse effects have led to withdrawal in many jurisdictions, and tachyphylaxis occurs. They cannot be recommended.
- Putative consequences or adverse effects of acid suppression [83]: most of these are based on retrospective analyses of heterogeneous populations and therefore show associations that may not be causal.
- Headache and diarrhea occur at a rate little different from that with placebo.
- Gastrointestinal infections [84]: a modestly increased risk of bacterial gastroenteritis and an association with increased risk of Clostridium difficile infection with PPI use.
- Respiratory tract infections: reports describing a modestly increased risk of community-acquired pneumonia with PPI use acknowledge the heterogeneity of the study outcomes, the absence of a clear pathophysiological basis, and the potential for unmeasured confounders.
- Low serum vitamin B12: not clinically significant.
- Hypomagnesemia — very rare, but documented with re-challenge studies.
- Cancer — no evidence of increased risk associated with PPI use per se.
- Osteoporosis, fractures — not likely or probable.
- Alarm features (see section 2.4):
- Check medication interactions.
- Rule out/treat other contributing conditions (constipation, exacerbating medications).
- Decide on the place of further investigations, “off-label” medications, and surgery.
4.3 GERD treatment in pregnancy
Table 6 Treatment options for GERD in pregnancy

4.4 Surgical interventions
Surgical intervention (usually fundoplication) in GERD patients is rarely indicated, but may be considered if there is a large hiatal hernia causing volume-related reflux symptoms and if there is evidence of aspiration or cardia dysfunction. Other indications may include noncompliance with medical therapy, side effects associated with medical therapy, esophagitis refractory to medical therapy, or persistent symptoms documented as being caused by refractory GERD [3].
- There is no evidence to support antireflux surgery for the sole indication of treating BE or preventing progression to early adenocarcinoma.
- Before antireflux surgery is considered, patients should be informed about the risk of long-term PPI use after surgery [85,86].
- Before deciding on antireflux surgery, one should consider checking PPI compliance and optimizing medical therapy [85].
The response to acid suppression (or neutralization) in patients with functional heartburn is by definition absent or minimal at best, and patients are at risk of being referred for surgical treatment for GERD. Hence, all patients with symptoms of GERD who are referred for surgery should undergo 24-hour pH monitoring to rule out functional heartburn [87]. They should also undergo esophageal manometry, a barium swallow, and EGD to rule out other possible diagnoses.
Surgical endoscopic antireflux techniques were developed starting in the late 1990s, but most have not survived, due to limited success [88]. There is still a lack of long- term outcome data for some procedures and new techniques, and these therapies should only be offered in the context of clinical trials.
4.5 Managing complications of GERD
Although the prognosis for patients with GERD is good, with up to 90% achieving good symptom control with optimum treatment, complications may occur — including bleeding, BE, strictures, ulceration, and malignancy.
Table 7 Recommendations for complications in GERD
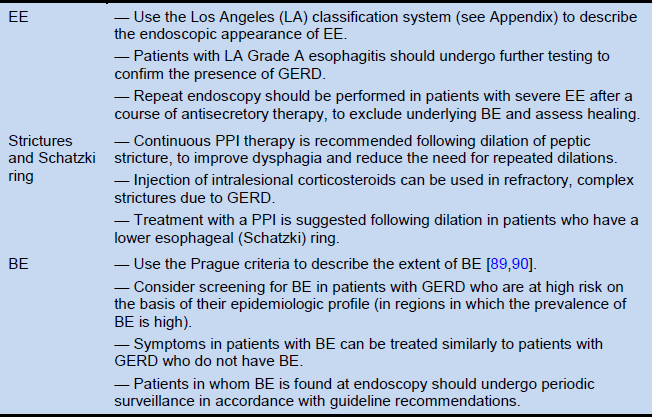
BE, Barrett’s esophagus; EE, erosive esophagitis; PPI, proton-pump inhibitor(s).
Note: These recommendations are based on the 2013 American College of Gastroenterology (ACG) Guidelines for managing complications of GERD [3]. The ACG guideline should be consulted for information about strength of evidence, evidence levels, and references. The Los Angeles classification is outlined in the Appendix (section 5.3, Table 10).
4.6 Cascades for the management of GERD
A thorough diagnostic evaluation of the patient’s history and a physical examination (see sections 3.1 and 3.2), including when symptoms occur (during the day or night, and in relation to meals) and the response (none, partial, or complete) to antacids, H2RAs, or PPIs are critical to provide the right guidance in resource-poor areas, in order to avoid unnecessary diagnostic investigations.
The Cascade shown in Table 8 assumes that there are no alarm features and no alternative, nongastrointestinal causes of the symptoms, that H. pylori infection has been sought and eradicated if indicated, and that NSAID use has been excluded as a cause of symptoms.
Table 8 Cascades: options in the management of GERD
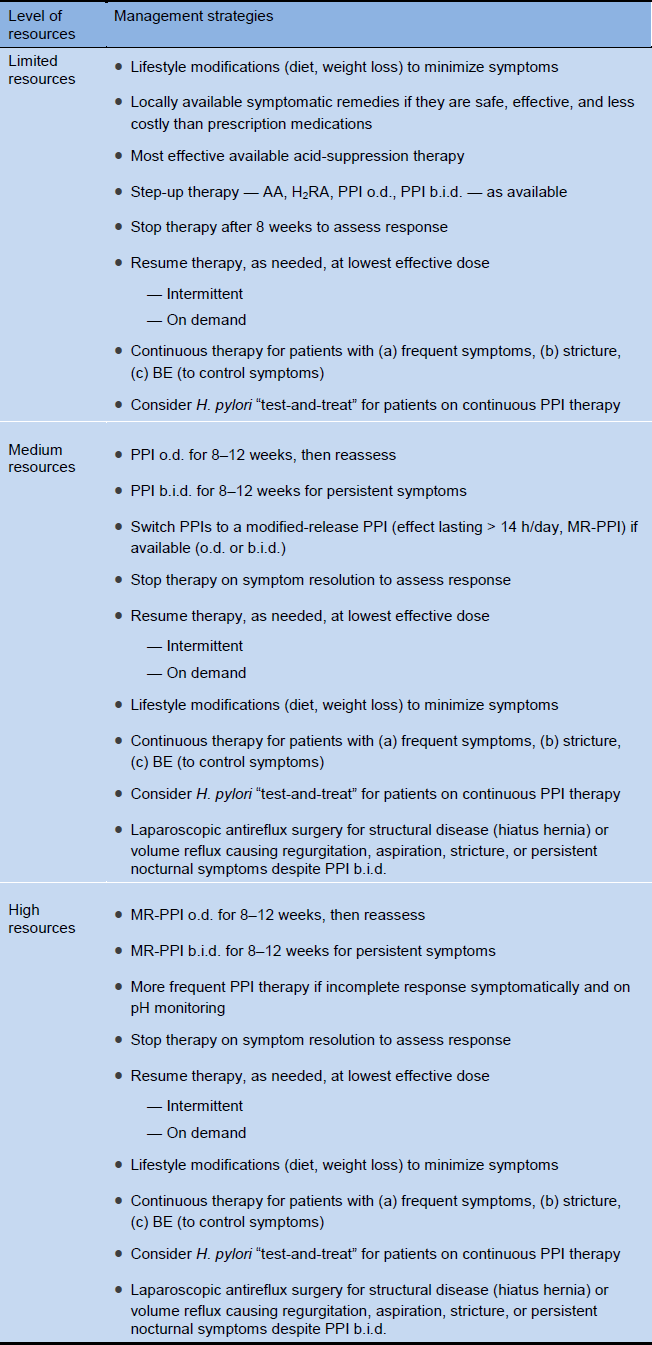
AA, alginate–antacid; BE, Barrett’s esophagus; b.i.d., bis in die (twice a day); H2RA, histamine H2-receptor antagonist; o.d., omni die (daily); MR-PPI, modified-release proton- pump inhibitor; PPI, proton-pump inhibitor.
5. Appendix
5.1 Abbreviations and definitions
Table 9 List of abbreviations (acronyms) and definitions

5.2 Gold standard guidelines on GERD
- 2013 American College of Gastroenterology guidelines for diagnosis and management:
Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol 2013;108:308–28; quiz 329. doi: 10.1038/ajg.2012.444. PMID: 23419381. National Guideline Clearinghouse NGC 009639.
- 2012 American College of Physicians Clinical Guidelines Committee best practice advice:
Shaheen NJ, Weinberg DS, Denberg TD, Chou R, Qaseem A, Shekelle P; Clinical Guidelines Committee of the American College of Physicians. Upper endoscopy for gastroesophageal reflux disease: best practice advice from the clinical guidelines committee of the American College of Physicians. Ann Intern Med 2012;157:808–16. doi: 10.7326/0003-4819-157-11-201212040-00008. PubMed PMID: 23208168
- 2011 American Gastroenterological Association medical position statement on the management of Barrett’s esophagus:
American Gastroenterological Association, Spechler SJ, Sharma P, Souza RF, Inadomi JM, Shaheen NJ. American Gastroenterological Association medical position statement on the management of Barrett’s esophagus. Gastroenterology 2011;140:1084–91. doi: 10.1053/j.gastro.2011.01.030. PubMed PMID: 21376940. National Guideline Clearinghouse NGC 008565.
- 2010 Brazilian GERD group consensus guidelines:
Moraes-Filho JP, Navarro-Rodriguez T, Barbuti R, Eisig J, Chinzon D, Bernardo W; Brazilian Gerd Consensus Group. Guidelines for the diagnosis and management of gastroesophageal reflux disease: an evidence-based consensus. Arq Gastroenterol 2010;47:99–115. PubMed PMID: 20520983.
- 2008 Asia–Pacific consensus update:
Fock KM, Talley NJ, Fass R, Goh KL, Katelaris P, Hunt R, et al. Asia–Pacific consensus on the management of gastroesophageal reflux disease: update. J Gastroenterol Hepatol 2008;23:8–22. doi: 10.1111/j.1440-1746.2007.05249.x. Erratum in: J Gastroenterol Hepatol 2008;23:504. PubMed PMID: 18171339.
- 2007 American Society for Gastrointestinal Endoscopy — role of endoscopy in the management of GERD:
Standards of Practice Committee, Lichtenstein DR, Cash BD, Davila R, Baron TH, Adler DG, et al. Role of endoscopy in the management of GERD. Gastrointest Endosc 2007;66:219–24. doi: 10.1016/j.gie.2007.05.027. PubMed PMID: 17643692.
- 2006 American Gastroenterological Association Institute medical position statement on endoscopic therapy in gastroesophageal reflux disease:
Falk GW, Fennerty MB, Rothstein RI. AGA Institute medical position statement on the use of endoscopic therapy for gastroesophageal reflux disease. Gastroenterology 2006;131:1313–4. PubMed PMID: 17030198.
- 2005 Canadian Association of Gastroenterology GERD Consensus Group, 2004 update:
Armstrong D, Marshall JK, Chiba N, Enns R, Fallone CA, Fass R, et al. Canadian Consensus Conference on the management of gastroesophageal reflux disease in adults — update 2004. Can J Gastroenterol 2005;19:15–35. PubMed PMID: 15685294.
- 2002 Gastroenterological Society of Australia guidelines for clinicians:
Katelaris P, Holloway R, Talley N, Gotley D, Williams S, Dent J, et al. Gastro- oesophageal reflux disease in adults: guidelines for clinicians. J Gastroenterol Hepatol 2002;17:825–33. doi: 10.1046/j.1440-1746.2002.02839.x. PubMed PMID: 12164956.
5.3 Los Angeles classification of erosive esophagitis
Table 10 Los Angeles classification of erosive esophagitis

5.4 Prague criteria for Barrett’s esophagus
The Prague criteria for BE provide a consensus-based endoscopic classification system that has undergone extensive internal and external validation by trained endoscopists. The Prague criteria provide a simple system for assessing the extent of Barrett’s esophagus, based on the length of distal esophagus involved circumferentially (C) and maximally (M) by Barrett’s epithelium with reference to the gastroesophageal junction, characterized by the proximal ends of the gastric mucosal folds and/or the lower esophageal sphincter “pinch.” These criteria have been shown to be identified and measured reliably by different endoscopists. The location of gastroesophageal landmarks is central to this classification and can also be reliably identified and located by different endoscopists. This standardized classification system enhances the ability of physicians to gauge the efficacy of treatments for BE in individual patients and the classification of patients with BE in clinical trials [89,90].
5.5 Regional epidemiologic data on GERD
Epidemiology of GERD in Japan
Michio Hongo
Table 11 Prevalence of GERD in eastern and southeastern Asia (data for Japan only)
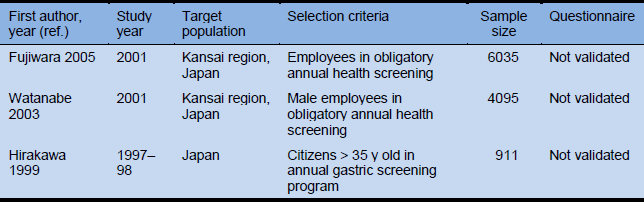
- Fujiwara Y, Higuchi K, Watanabe Y, Shiba M, Watanabe T, Tominaga K, et al. Prevalence of gastroesophageal reflux disease and gastroesophageal reflux disease symptoms in Japan. J Gastroenterol Hepatol 2005;20:26–9.
Of the 6035 eligible patients, 2662 (44.1%) reported having had heartburn and/or acid regurgitation during the previous year: 124 (2.1%) daily, 275 (4.6%) twice per week, 773 (12.8%) twice per month, and 1490 (24.7%) less than twice per month. Three hundred and ninety-nine (6.6%) patients were diagnosed as having GERD, and there was no relationship between the prevalence of GERD and either sex or age.
- Fujimoto K. Review article: prevalence and epidemiology of gastro- oesophageal reflux disease in Japan. Aliment Pharmacol Ther 2004;20 Suppl 8:5–8.
Endoscopic studies show that the overall prevalence of reflux esophagitis among the adult population in Japan is in the region of 14–16%.
- Fujimoto K, Iwakiri R, Okamoto K, Oda K, Tanaka A, Tsunada S, et al. Characteristics of gastroesophageal reflux disease in Japan: increased prevalence in elderly women. J Gastroenterol 2003;38 Suppl 15:3–6.
The ratios of patients with each complaint relative to all patients were as follows: heartburn, 27.0%; dysphagia, 16.9%; odynophagia, 19.2%; acid regurgitation, 7.1%. The proportions of each grade were grade A, 9.6%; grade B, 4.6%; and grade C + D, 2.0%.
- Wong BC, Kinoshita Y. Systematic review on epidemiology of gastroesophageal reflux disease in Asia. Clin Gastroenterol Hepatol 2006;4:398–407.
The reported population prevalence of GERD in eastern Asia ranged from 2.5% to 6.7% for at least weekly symptoms of heartburn and/or acid regurgitation and may be increasing. In case studies, the prevalence of reflux esophagitis ranged from 3.4% to 16.3%.
Table 12 Prevalence of esophagitis in eastern and southeastern Asia (data for Japan only)

- Fujiwara Y, Arakawa T. Epidemiology and clinical characteristics of GERD in the Japanese population. J Gastroenterol 2009;44:518–34.
- Yamagishi H, Koike T, Ohara S, Kobayashi S, Ariizumi K, Abe Y, et al. Prevalence of gastroesophageal reflux symptoms in a large unselected general population in Japan. World J Gastroenterol 2008;14:1358–64.
The prevalence of typical GERD symptoms (heartburn) was high, at about 20% of the Japanese population, and the frequency was especially high in women in the 60–89-year-old age group.
- Kinoshita Y, Adachi K, Hongo M, Haruma K. Systematic review of the epidemiology of gastroesophageal reflux disease in Japan. J Gastroenterol 2011;46:1092–103.
Seven studies reported that the prevalence of at least weekly symptoms was 6.5–9.5%, a figure approaching that reported in Western populations (10–20%).
Epidemiology of GERD in India
Shobna Bhatia
The prevalence of weekly GERD symptoms has been reported in various studies from India and ranges from 7.6% to 19%.
Table 13 Prevalence of weekly GERD symptoms in India
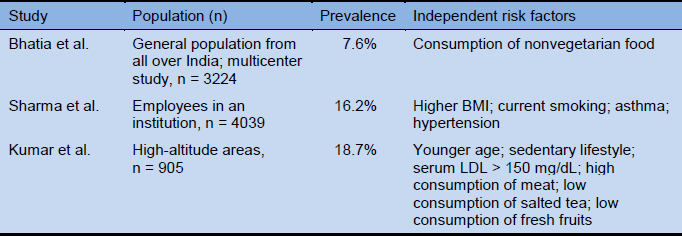
BMI, body mass index; LDL, low-density lipoprotein.
- Bhatia SJ, Reddy DN, Ghoshal UC, Jayanthi V, Abraham P, Choudhuri G, et al. Epidemiology and symptom profile of gastroesophageal reflux in the Indian population: report of the Indian Society of Gastroenterology Task Force. Indian J Gastroenterol 2011;30:118–27.
- Sharma PK, Ahuja V, Madan K, Gupta S, Raizada A, Sharma MP. Prevalence, severity, and risk factors of symptomatic gastroesophageal reflux disease among employees of a large hospital in Northern India. Indian J Gastroenterol 2011;30:128–34.
- Kumar S, Sharma S, Norboo T, Dolma D, Norboo A, Stobdan T, et al. Population based study to assess prevalence and risk factors of gastroesophageal reflux disease in a high altitude area. Indian J Gastroenterol 2011;30:135–43.
- Chowdhury SD, George G, Ramakrishna K, Balamurugan R, Mechenro J, Ramakrishna BS. Prevalence and associations of gastro esophageal reflux disease: a community study in south India [abstract]. Gastroenterology 2015;148:S-403–4.
Epidemiology of GERD in Brazil
Joachim Moraes-Filho
The prevalence of heartburn (11.9%) is relatively high in the urban population in Brazil, although lower than the figures reported from other countries. Heartburn and GERD have a higher prevalence among women, and both are related to food intake, fatty and spicy foods; GERD is more prevalent in individuals over the age of 35.
- Moraes-Filho JP, Chinzon D, Eisig JN, Hashimoto CL, Zaterka S. Prevalence of heartburn and gastroesophageal reflux disease in the urban Brazilian population. Arq Gastroenterol 2005;42:122–7.
Epidemiology of GERD in Asan-si, Korea
Young-Seok Cho
The prevalence of GERD among the population of the city of Asan-si in Korea was 3.5%. Heartburn and acid regurgitation were significantly associated with chest pain, dysphagia, globus sensation, hoarseness, and asthma.
- Cho YS, Choi MG, Jeong JJ, Chung WC, Lee IS, Kim SW, et al. Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Asan-si, Korea. Am J Gastroenterol 2005;100:747–53.
Epidemiology of GERD in Argentina
Graciela Salis
In Argentina, typical symptoms of gastroesophageal reflux are highly prevalent at the national level (the prevalence of frequent reflux symptoms was 23.0% (95% CI, 20.1 to 25.9) and the prevalence of GERD was 11.9% (95% CI, 9.6 to 14.1), and frequent gastroesophageal reflux symptoms are significantly associated with dysphagia, globus, and noncardiac chest pain.
- Chiocca JC, Olmos JA, Salis GB, Soifer LO, Higa R, Marcolongo M, et al. Prevalence, clinical spectrum and atypical symptoms of gastro-oesophageal reflux in Argentina: a nationwide population-based study. Aliment Pharmacol Ther 2005;22:331–42.
Epidemiology of GERD in Russia
Leonid Lazebnik
The population-based MEGRE trial, conducted on the basis of internationally recognized methodology in six cities in Russia, showed that the prevalence of GERD is 13.3%. Most of the patients pay little attention to the symptoms, do not seek medical advice, and therefore do not receive any adequate treatment. Heartburn occurred in 47.5% of the responders: frequently in 9% and rarely in 38.5%. Regurgitation occurred in 42.9% of the patients: frequently in 7.6%, rarely in 35.3%.
- Lazebnik LB, Masharova AA, Bordin DS, Vasil’ev IuV, Tkachenko EI, Abdulkhakov RA, et al. [Results of a multicenter trial “Epidemiology of Gastroesophageal Reflux Disease in Russia” (MEGRE)]. Ter Arkh 2011;83:45–50. [Article in Russian.]
6. References
- Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R, Global Consensus Group. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol 2006;101:1900–20; quiz 1943.
- Moayyedi P, Talley NJ. Gastro-oesophageal reflux disease. Lancet 2006;367:2086–100.
- Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol 2013;108:308–28; quiz 329.
- Dent J, Becher A, Sung J, Zou D, Agréus L, Bazzoli F. Systematic review: patterns of reflux- induced symptoms and esophageal endoscopic findings in large-scale surveys. Clin Gastroenterol Hepatol 2012;10:863–73.e3.
- Modlin IM, Hunt RH, Malfertheiner P, Moayyedi P, Quigley EM, Tytgat GNJ, et al. Diagnosis and management of non-erosive reflux disease—the Vevey NERD Consensus Group. Digestion 2009;80:74–88.
- Hunt R, Quigley E, Abbas Z, Eliakim A, Emmanuel A, Goh KL, et al. Coping with common gastrointestinal symptoms in the community: a global perspective on heartburn, constipation, bloating, and abdominal pain/discomfort, May 2013. J Clin Gastroenterol 2014;48:567–78.
- Modlin IM, Moss SF. Symptom evaluation in gastroesophageal reflux disease. J Clin Gastroenterol 2008;42:558–63.
- Dent J, Armstrong D, Delaney B, Moayyedi P, Talley NJ, Vakil N. Symptom evaluation in reflux disease: workshop background, processes, terminology, recommendations, and discussion outputs. Gut 2004;53 Suppl 4:iv1–24.
- El-Serag HB, Sweet S, Winchester CC, Dent J. Update on the epidemiology of gastro- oesophageal reflux disease: a systematic review. Gut 2014;63:871–80.
- Henke CJ, Levin TR, Henning JM, Potter LP. Work loss costs due to peptic ulcer disease and gastroesophageal reflux disease in a health maintenance organization. Am J Gastroenterol 2000;95:788–92.
- Liker H, Hungin P, Wiklund I. Managing gastroesophageal reflux disease in primary care: the patient perspective. J Am Board Fam Pract 2005;18:393–400.
- Revicki DA, Wood M, Maton PN, Sorensen S. The impact of gastroesophageal reflux disease on health-related quality of life. Am J Med 1998;104:252–8.
- Veldhuyzen van Zanten SJ, Flook N, Chiba N, Armstrong D, Barkun A, Bradette M, et al. An evidence-based approach to the management of uninvestigated dyspepsia in the era of Helicobacter pylori. Canadian Dyspepsia Working Group. CMAJ 2000;162(12 Suppl):S3–23.
- El-Serag H. The association between obesity and GERD: a review of the epidemiological evidence. Dig Dis Sci 2008;53:2307–12.
- Goh KL. Changing epidemiology of gastroesophageal reflux disease in the Asian-Pacific region: an overview. J Gastroenterol Hepatol 2004;19 Suppl 3:S22–5.
- El-Serag HB, Satia JA, Rabeneck L. Dietary intake and the risk of gastro-oesophageal reflux disease: a cross sectional study in volunteers. Gut 2005;54:11–7.
- Fass R, Quan SF, O’Connor GT, Ervin A, Iber C. Predictors of heartburn during sleep in a large prospective cohort study. Chest 2005;127:1658–66.
- DiBaise JK. A randomized, double-blind comparison of two different coffee-roasting processes on development of heartburn and dyspepsia in coffee-sensitive individuals. Dig Dis Sci 2003;48:652–6.
- Akiyama T, Inamori M, Iida H, Mawatari H, Endo H, Hosono K, et al. Alcohol consumption is associated with an increased risk of erosive esophagitis and Barrett’s epithelium in Japanese men. BMC Gastroenterol 2008;8:58.
- Gunasekaran TS, Dahlberg M, Ramesh P, Namachivayam G. Prevalence and associated features of gastroesophageal reflux symptoms in a Caucasian-predominant adolescent school population. Dig Dis Sci 2008;53:2373–9.
- Eslick GD, Talley NJ. Gastroesophageal reflux disease (GERD): risk factors, and impact on quality of life—a population-based study. J Clin Gastroenterol 2009;43:111–7.
- Nilsson M, Johnsen R, Ye W, Hveem K, Lagergren J. Lifestyle related risk factors in the aetiology of gastro-oesophageal reflux. Gut 2004;53:1730–5.
- Moraes-Filho JPP, Navarro-Rodriguez T, Eisig JN, Barbuti RC, Chinzon D, Quigley EMM. Comorbidities are frequent in patients with gastroesophageal reflux disease in a tertiary health care hospital. Clin São Paulo Braz 2009;64:785–90.
- Keller J, Frederking D, Layer P. The spectrum and treatment of gastrointestinal disorders during pregnancy. Nat Clin Pract Gastroenterol Hepatol 2008;5:430–43.
- Richter JE. Review article: the management of heartburn in pregnancy. Aliment Pharmacol Ther 2005;22:749–57.
- Marrero JM, Goggin PM, de Caestecker JS, Pearce JM, Maxwell JD. Determinants of pregnancy heartburn. Br J Obstet Gynaecol 1992;99:731–4.
- Habr F, Raker C, Lin CL, Zouein E, Bourjeily G. Predictors of gastroesophageal reflux symptoms in pregnant women screened for sleep disordered breathing: a secondary analysis. Clin Res Hepatol Gastroenterol 2013;37:93–9.
- Nazer D, Thomas R, Tolia V. Ethnicity and gender related differences in extended intraesophageal pH monitoring parameters in infants: a retrospective study. BMC Pediatr 2005;5:24.
- Yamaguchi T, Sugimoto T, Yamada H, Kanzawa M, Yano S, Yamauchi M, et al. The presence and severity of vertebral fractures is associated with the presence of esophageal hiatal hernia in postmenopausal women. Osteoporos Int USA 2002;13:331–6.
- Watanabe A, Iwakiri R, Yamaguchi D, Higuchi T, Tsuruoka N, Miyahara K, et al. Risk factors for resistance to proton pump inhibitor maintenance therapy for reflux esophagitis in Japanese women over 60 years. Digestion 2012;86:323–8.
- Akiyama T, Inamori M, Akimoto K, Iida H, Mawatari H, Endo H, et al. Gender differences in the age-stratified prevalence of erosive esophagitis and Barrett’s epithelium in Japan. Hepatogastroenterology 2009;56:144–8.
- Dent J, Vakil N, Jones R, Bytzer P, Schöning U, Halling K, et al. Accuracy of the diagnosis of GORD by questionnaire, physicians and a trial of proton pump inhibitor treatment: the Diamond Study. Gut 2010;59:714–21.
- Thomson ABR, Barkun AN, Armstrong D, Chiba N, White RJ, Daniels S, et al. The prevalence of clinically significant endoscopic findings in primary care patients with uninvestigated dyspepsia: the Canadian Adult Dyspepsia Empiric Treatment — Prompt Endoscopy (CADET- PE) study. Aliment Pharmacol Ther 2003;17:1481–91.
- Boeckxstaens GE, Smout A. Systematic review: role of acid, weakly acidic and weakly alkaline reflux in gastro-oesophageal reflux disease. Aliment Pharmacol Ther 2010;32:334–43.
- Atkins D, Briss PA, Eccles M, Flottorp S, Guyatt GH, Harbour RT, et al. Systems for grading the quality of evidence and the strength of recommendations II: pilot study of a new system. BMC Health Serv Res 2005;5:25.
- Bruley des Varannes S, Cestari R, Usova L, Triantafyllou K, Alvarez Sanchez A, Keim S, et al. Classification of adults suffering from typical gastroesophageal reflux disease symptoms: contribution of latent class analysis in a European observational study. BMC Gastroenterol 2014;14:112.
- DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey LM, editors. Pharmacotherapy: a pathophysiologic approach. 9th ed. New York: McGraw-Hill; 2014.
- Vakil NB, Traxler B, Levine D. Dysphagia in patients with erosive esophagitis: prevalence, severity, and response to proton pump inhibitor treatment. Clin Gastroenterol Hepatol 2004;2:665–8.
- Malfertheiner P, Nocon M, Vieth M, Stolte M, Jaspersen D, Koelz HR, et al. Evolution of gastro-oesophageal reflux disease over 5 years under routine medical care—the ProGERD study. Aliment Pharmacol Ther 2012;35:154–64.
- Corley DA, Kubo A. Body mass index and gastroesophageal reflux disease: a systematic review and meta-analysis. Am J Gastroenterol 2006;101:2619–28.
- Lundell LR, Dent J, Bennett JR, Blum AL, Armstrong D, Galmiche JP, et al. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut 1999;45:172–80.
- Navarro-Rodriguez T, Fass R. Functional heartburn, nonerosive reflux disease, and reflux esophagitis are all distinct conditions—a debate: pro. Curr Treat Options Gastroenterol 2007;10:294–304.
- Ronkainen J, Talley NJ, Storskrubb T, Johansson SE, Lind T, Vieth M, et al. Erosive esophagitis is a risk factor for Barrett’s esophagus: a community-based endoscopic follow-up study. Am J Gastroenterol 2011;106:1946–52.
- Kuipers EJ. Barrett esophagus and life expectancy: implications for screening? Gastroenterol Hepatol 2011;7:689–91.
- Lagergren J, Bergström R, Nyrén O. Association between body mass and adenocarcinoma of the esophagus and gastric cardia. Ann Intern Med 1999;130:883–90.
- Malagelada J, Bazzoli F, Boeckxstaens G, De Looze D, Fried M, Kahrilas P, et al. World Gastroenterology Organisation Global Guidelines. Dysphagia [Internet]. Milwaukee, WI: World Gastroenterology Organisation; 2014 [accessed 2015 Dec 8]. Available from: http://www.worldgastroenterology.org/guidelines/global-guidelines/dysphagia/dysphagia- english.
- Moraes-Filho J, Cecconello I, Gama-Rodrigues J, Castro L, Henry MA, Meneghelli UG, et al. Brazilian consensus on gastroesophageal reflux disease: proposals for assessment, classification, and management. Am J Gastroenterol 2002;97:241–8.
- Hirano I, Richter JE, Practice Parameters Committee of the American College of Gastroenterology. ACG practice guidelines: esophageal reflux testing. Am J Gastroenterol 2007;102:668–85.
- Hemmink GJM, Bredenoord AJ, Weusten BLAM, Monkelbaan JF, Timmer R, Smout AJPM. Esophageal pH-impedance monitoring in patients with therapy-resistant reflux symptoms: “on” or “off” proton pump inhibitor? Am J Gastroenterol 2008;103:2446–53.
- Malfertheiner P, Megraud F, O’Morain CA, Atherton J, Axon ATR, Bazzoli F, et al. Management of Helicobacter pylori infection—the Maastricht IV/ Florence Consensus Report. Gut 2012;61:646–64.
- Sugano K, Tack J, Kuipers EJ, Graham DY, El-Omar EM, Miura S, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut 2015;64:1353–67.
- Yaghoobi M, Farrokhyar F, Yuan Y, Hunt RH. Is there an increased risk of GERD after Helicobacter pylori eradication? A meta-analysis. Am J Gastroenterol 2010;105:1007–13; quiz 1006, 1014.
- Chiba N, Van Zanten SJOV, Sinclair P, Ferguson RA, Escobedo S, Grace E. Treating Helicobacter pylori infection in primary care patients with uninvestigated dyspepsia: the Canadian adult dyspepsia empiric treatment—Helicobacter pylori positive (CADET-Hp) randomised controlled trial. BMJ 2002;324:1012–6.
- Kuipers EJ, Lundell L, Klinkenberg-Knol EC, Havu N, Festen HP, Liedman B, et al. Atrophic gastritis and Helicobacter pylori infection in patients with reflux esophagitis treated with omeprazole or fundoplication. N Engl J Med 1996;334:1018–22.
- Lundell L, Vieth M, Gibson F, Nagy P, Kahrilas PJ. Systematic review: the effects of long-term proton pump inhibitor use on serum gastrin levels and gastric histology. Aliment Pharmacol Ther 2015;42:649–63.
- Chen SL, Gwee KA, Lee JS, Miwa H, Suzuki H, Guo P, et al. Systematic review with meta- analysis: prompt endoscopy as the initial management strategy for uninvestigated dyspepsia in Asia. Aliment Pharmacol Ther 2015;41:239–52.
- Chander B, Hanley-Williams N, Deng Y, Sheth A. 24 Versus 48-hour bravo pH monitoring. J Clin Gastroenterol 2012;46:197–200.
- Liacouras CA, Furuta GT, Hirano I, Atkins D, Attwood SE, Bonis PA, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol 2011;128:3–20.e6; quiz 21–2.
- Zimmerman J, Hershcovici T. Bowel symptoms in nonerosive gastroesophageal reflux disease: nature, prevalence, and relation to acid reflux. J Clin Gastroenterol 2008;42:261–5.
- Festi D, Scaioli E, Baldi F, Vestito A, Pasqui F, Di Biase AR, et al. Body weight, lifestyle, dietary habits and gastroesophageal reflux disease. World J Gastroenterol 2009;15:1690–701.
- de Bortoli N, Guidi G, Martinucci I, Savarino E, Imam H, Bertani L, et al. Voluntary and controlled weight loss can reduce symptoms and proton pump inhibitor use and dosage in patients with gastroesophageal reflux disease: a comparative study. Dis Esophagus 2014 Dec 17 [Epub ahead of print].
- Piche T, des Varannes SB, Sacher-Huvelin S, Holst JJ, Cuber JC, Galmiche JP. Colonic fermentation influences lower esophageal sphincter function in gastroesophageal reflux disease. Gastroenterology 2003;124:894–902.
- [No authors listed.] An evidence-based appraisal of reflux disease management—the Genval Workshop Report. Gut 1999;44 Suppl 2:S1–16.
- Becher A, Dent J. Systematic review: ageing and gastro-oesophageal reflux disease symptoms, oesophageal function and reflux oesophagitis. Aliment Pharmacol Ther 2011;33:442–54.
- Knuff TE, Benjamin SB, Worsham GF, Hancock JE, Castell DO. Histologic evaluation of chronic gastroesophageal reflux. An evaluation of biopsy methods and diagnostic criteria. Dig Dis Sci 1984;29:194–201.
- Johnsson F, Joelsson B, Gudmundsson K, Greiff L. Symptoms and endoscopic findings in the diagnosis of gastroesophageal reflux disease. Scand J Gastroenterol 1987;22:714–8.
- Johnston BT, Troshinsky MB, Castell JA, Castell DO. Comparison of barium radiology with esophageal pH monitoring in the diagnosis of gastroesophageal reflux disease. Am J Gastroenterol 1996;91:1181–5.
- Peng S, Xiong LS, Xiao YL, Lin JK, Wang AJ, Zhang N, et al. Prompt upper endoscopy is an appropriate initial management in uninvestigated chinese patients with typical reflux symptoms. Am J Gastroenterol 2010;105:1947–52.
- Wang C, Hunt RH. Medical management of gastroesophageal reflux disease. Gastroenterol Clin North Am 2008;37:879–99, ix.
- Tytgat GN, McColl K, Tack J, Holtmann G, Hunt RH, Malfertheiner P, et al. New algorithm for the treatment of gastro-oesophageal reflux disease. Aliment Pharmacol Ther 2008;27:249–56.
- Sheikh I, Waghray A, Waghray N, Dong C, Wolfe MM. Consumer use of over-the-counter proton pump inhibitors in patients with gastroesophageal reflux disease. Am J Gastroenterol 2014;109:789–94.
- Person E, Rife C, Freeman J, Clark A, Castell DO. A novel sleep positioning device reduces gastroesophageal reflux: a randomized controlled trial. J Clin Gastroenterol 2015;49:655–9.
- Boardman HF, Heeley G. The role of the pharmacist in the selection and use of over-the-counter proton-pump inhibitors. Int J Clin Pharm 2015;37:709–16.
- Berardi RR, American Pharmacists Association, editors. Handbook of nonprescription drugs: an interactive approach to self-care. 16th ed. Washington, DC: American Pharmacists Association; 2009.
- Weijenborg PW, Cremonini F, Smout AJPM, Bredenoord AJ. PPI therapy is equally effective in well-defined non-erosive reflux disease and in reflux esophagitis: a meta-analysis. Neurogastroenterol Motil 2012;24:747–57, e350.
- Bell NJ, Burget D, Howden CW, Wilkinson J, Hunt RH. Appropriate acid suppression for the management of gastro-oesophageal reflux disease. Digestion 1992;51 Suppl 1:59–67.
- Hunt RH. Importance of pH control in the management of GERD. Arch Intern Med 1999;159:649–57.
- Howden CW, Kahrilas PJ. Editorial: just how “difficult” is it to withdraw PPI treatment? Am J Gastroenterol 2010;105:1538–40.
- Niv Y. Gradual cessation of proton pump inhibitor (PPI) treatment may prevent rebound acid secretion, measured by the alkaline tide method, in dyspepsia and reflux patients. Med Hypotheses 2011;77:451–2.
- Fass R, Sifrim D. Management of heartburn not responding to proton pump inhibitors. Gut 2009;58:295–309.
- Richter JE. The patient with refractory gastroesophageal reflux disease. Dis Esophagus 2006;19:443–7.
- Dal-Paz K, Moraes-Filho JP, Navarro-Rodriguez T, Eisig JN, Barbuti R, Quigley EMM. Low levels of adherence with proton pump inhibitor therapy contribute to therapeutic failure in gastroesophageal reflux disease. Dis Esophagus 2012;25:107–13.
- Sheen E, Triadafilopoulos G. Adverse effects of long-term proton pump inhibitor therapy. Dig Dis Sci 2011;56:931–50.
- Leonard J, Marshall JK, Moayyedi P. Systematic review of the risk of enteric infection in patients taking acid suppression. Am J Gastroenterol 2007;102:2047–56; quiz 2057.
- Lødrup A, Pottegård A, Hallas J, Bytzer P. Use of proton pump inhibitors after antireflux surgery: a nationwide register-based follow-up study. Gut 2014;63:1544–9.
- Madan A, Minocha A. Despite high satisfaction, majority of gastro-oesophageal reflux disease patients continue to use proton pump inhibitors after antireflux surgery. Aliment Pharmacol Ther 2006;23:601–5.
- Thomas V, Rangan K, Kumar S. Occurrence of functional heartburn in patients with symptoms of gastroesophageal reflux disease (GERD) not responding to proton pump inhibitors (PPI) [abstract] 2011;106(Suppl 2):S25.
- Fuchs KH, Babic B, Breithaupt W, Dallemagne B, Fingerhut A, Furnee E, et al. EAES recommendations for the management of gastroesophageal reflux disease. Surg Endosc 2014;28:1753–73.
- Alvarez Herrero L, Curvers WL, van Vilsteren FGI, Wolfsen H, Ragunath K, Wong Kee Song LM, et al. Validation of the Prague C&M classification of Barrett’s esophagus in clinical practice. Endoscopy 2013;45:876–82.
- Sharma P, Dent J, Armstrong D, Bergman JJGHM, Gossner L, Hoshihara Y, et al. The development and validation of an endoscopic grading system for Barrett’s esophagus: the Prague C & M criteria. Gastroenterology 2006;131:1392–9.

















