1. Introduction
Constipation is a chronic problem in many patients all over the world. In some groups of patients such as the elderly, constipation is a significant health-care problem, but in the majority of cases chronic constipation is an aggravating, but not life-threatening or debilitating, complaint that can be managed in primary care with cost-effective control of symptoms.
The terminology associated with constipation is problematic. There are two pathophysiologies, which differ in principle but overlap: disorders of transit and evacuation disorders. The first can arise secondary to the second, and the second can sometimes follow from the first.
This guideline focuses on adult patients and does not specifically discuss children or special groups of patients (such as those with spinal cord injury).
1.1 Cascades—a resource-sensitive approach
A gold standard approach is feasible for regions and countries in which the full range of diagnostic tests and medical treatment options is available for the management of all types and subtypes of constipation.
Cascade: a hierarchical set of diagnostic, therapeutic, and management options for dealing with risk and disease, ranked according to the resources available.
2. Definition and pathogenesis
The word “constipation” has several meanings, and the way it is used may differ not only between patients but also between different cultures and regions. In a Swedish population study, it was found that a need to take laxatives was the most common conception of constipation (57% of respondents). In the same study, women (41%) were twice as likely as men (21%) to regard infrequent bowel motions as representing constipation, whereas equal proportions of men and women regarded hard stools (43%), straining during bowel movements (24%), and pain when passing a motion (23%) as representing constipation. Depending on various factors—the diagnostic definition, demographic factors, and group sampling—constipation surveys show a prevalence of between 1% and more than 20% in Western populations. In studies of the elderly population, up to 20% of community-dwelling individuals and 50% of institutionalized elderly persons reported symptoms.
Functional constipation is generally defined as a disorder characterized by persistent difficult or seemingly incomplete defecation and/or infrequent bowel movements (once every 3–4 days or less) in the absence of alarm symptoms or secondary causes. Differences in the medical definition and variations in the reported symptoms make it difficult to provide reliable epidemiologic data.
2.1 Pathogenesis and risk factors
Functional constipation can have many different causes, ranging from changes in diet, physical activity, or lifestyle to primary motor dysfunctions due to colonic myopathy or neuropathy. Constipation can also be secondary to evacuation disorder. Evacuation disorder may be associated with a paradoxical anal contraction or involuntary anal spasm, which may be an acquired behavioral disorder of defecation in two-thirds of patients.
Table 1 Pathophysiology of functional constipation
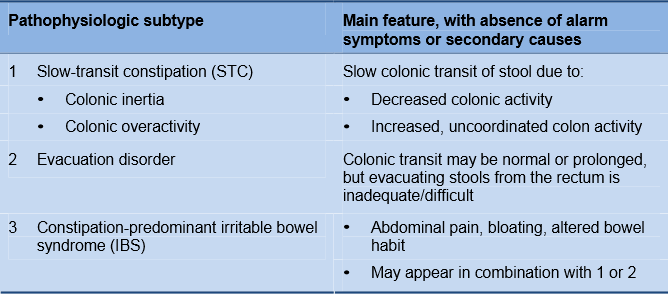
While physical exercise and a high-fiber diet may be protective, the following factors increase the risk of constipation (the association may not be causative):
- Aging (but constipation is not a physiological consequence of normal aging)
- Depression
- Inactivity
- Low calorie intake
- Low income and low education level
- Number of medications being taken (independent adverse effect profiles)
- Physical and sexual abuse
- Female sex—higher incidence self-reported constipation in women
2.2 Associated conditions and medications
Table 2 Possible causes and constipation-associated conditions
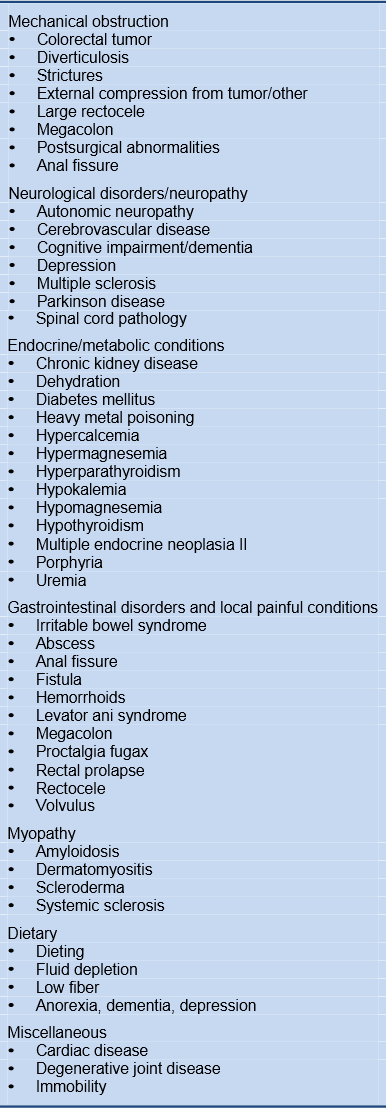
Table 3 Medications associated with constipation

3. Diagnosis
Constipation is a common condition, and although a minority of patients seek medical care, in the United States alone this accounts for several million physician visits per year, while in the United Kingdom more than 13 million general practitioner prescriptions were written for laxatives in 2006. Gastrointestinal specialist help should focus on efficiently applying health-care resources by identifying those patients who are likely to benefit from specialized diagnostic evaluation and treatment.
3.1 Diagnostic criteria for functional constipation
An international panel of experts developed uniform criteria for the diagnosis of constipation—the Rome III criteria.
Table 4 Rome III criteria for functional constipation

3.2 Patient evaluation
The medical history and physical examination in constipation patients should focus on identifying possible causative conditions and alarm symptoms.
- Stool consistency. This is regarded as a better indicator of colon transit than stool frequency (Fig. 1).
Fig. 1 The Bristol Stool Form Scale: a measure to assist patients in reporting on stool consistency (Reproduced with permission from Lewis SJ and Heaton KW, et al, Scandinavian Journal of Gastroenterology 1997;32:920–4). ©1997 Informa Healthcare
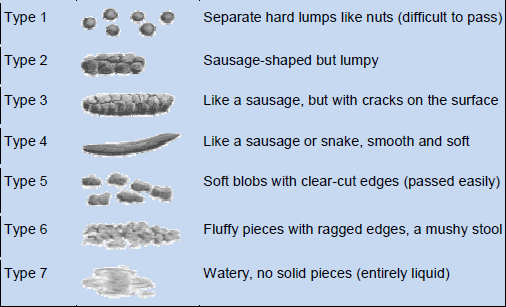
- Patient’s description of constipation symptoms; symptom diary:
- Bloating, pain, malaise
- Nature of stools
- Bowel movements
- Prolonged/excessive straining
- Unsatisfactory defecation
- Laxative use, past and present; frequency and dosage
- Current conditions, medical history, recent surgery, psychiatric illness
- Patient’s lifestyle, dietary fiber, and fluid intake
- Use of suppositories or enemas, other medications (prescription or over-thecounter)
- Physical examination:
- Gastrointestinal mass
- Anorectal inspection:
- Fecal impaction
- Stricture, rectal prolapse, rectocele
- Paradoxical or nonrelaxing puborectalis activity
- Rectal mass
- If indicated: blood tests—biochemical profile, complete blood count, calcium, glucose, and thyroid function
3.3 Alarm symptoms
Table 5 Alarm symptoms in constipation
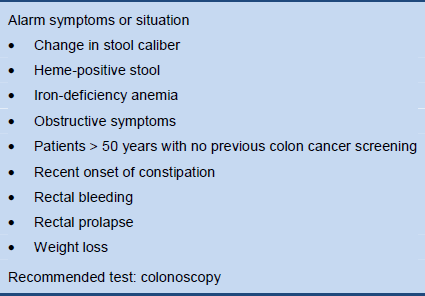
3.4 Indications for screening tests
Laboratory studies, imaging or endoscopy, and function tests are only indicated in patients with severe chronic constipation or alarm symptoms.
Table 6 Physiologic tests for chronic constipation (reproduced with permission from Rao SS, Gastrointest Endosc Clin N Am 2009;19:117–39)
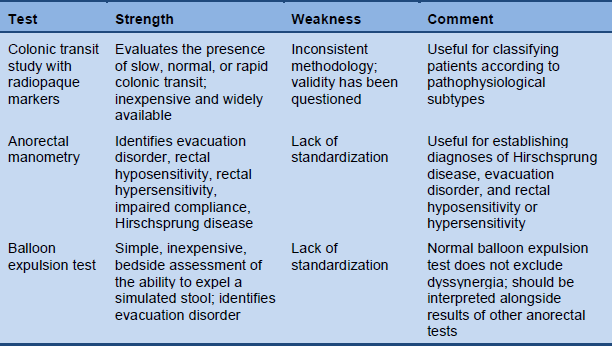
3.5 Transit measurement
The 5-day marker retention study is a simple method for measuring colonic transit. Markers are ingested on one occasion and remaining markers are counted on a plain abdominal radiograph 120 hours later. If more than 20% of the markers remain in the colon, transit is delayed. Distal accumulation of markers may indicate an evacuation disorder, and in typical cases of slow-transit constipation almost all markers remain and markers are seen in both the right and the left colon.
Several companies produce markers, but markers can also be made from a patientsafe radiopaque tube by cutting it into small pieces (2–3 mm in length). A suitable number of markers (20–24) can be placed in gelatin capsules to facilitate ingestion.
3.6 Clinical evaluation
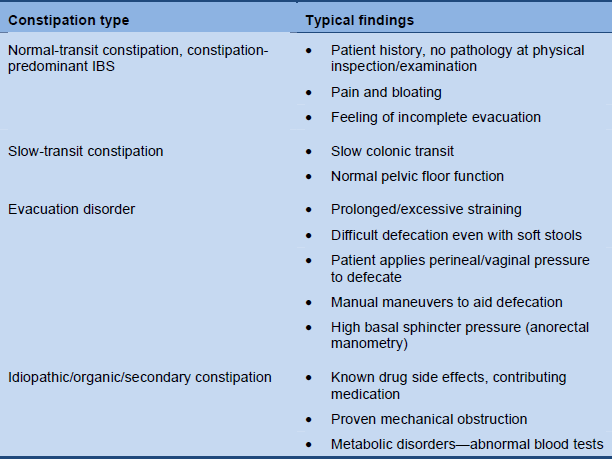
Classification of the patient’s constipation should be possible on the basis of the medical history and appropriate examination and testing.
Table 7 Constipation categories based on clinical evaluation
3.7 Cascade options for investigating severe and treatment-refractory constipation
Level 1—limited resources
- Medical history and general physical examination
- Anorectal examination, 1-week bowel habit diary card
- Transit study using radiopaque markers
- Balloon expulsion test
Level 2—medium resources
- Medical history and general physical examination
- Anorectal examination, 1-week bowel habit diary card
- Transit study using radiopaque markers
- Balloon expulsion test or defecography
Level 3—extensive resources
- Medical history and general physical examination
- Anorectal examination, 1-week bowel habit diary card
- Transit study using radio-opaque markers
- Defecography or magnetic resonance (MR) proctography
- Anorectal manometry
- Sphincter electromyography (EMG)
4. Treatment
4.1 Scheme for general management of constipation
Table 8 General management of constipation
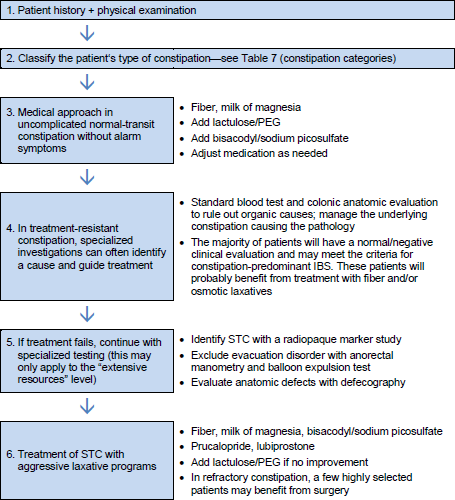
IBS, irritable bowel syndrome; PEG, polyethylene glycol; STC, slow-transit constipation.
4.2 Symptomatic approach
If organic and secondary constipation have been evaluated and excluded, most cases can be managed adequately with a symptomatic approach.
- A graded approach to treatment is based on recommending changes in lifestyle and diet, stopping or reducing medications that cause constipation, and administering fiber supplementation or other bulk-forming agents. A gradual increase in fiber (either as standardized supplements or incorporated in the diet) and fluid intake is generally recommended.
- The second step in the graded approach is to add osmotic laxatives. The best evidence is for the use of polyethylene glycol, but there is also good evidence for lactulose. The new drugs lubiprostone and linaclotide act by stimulating ileal secretion and thus increasing fecal water. Prucalopride is also approved in many countries and in Europe.
- The third step includes stimulant laxatives, enemas, and prokinetic drugs. Stimulant laxatives can be given orally or rectally to stimulate colorectal motor activity. Prokinetic drugs are also meant to increase the propulsive activity of the colon, but in contrast to stimulant laxatives, which should only be taken occasionally, they are designed to be taken daily.
4.3 Diet and supplements
- Dietary modification may consist of a high-fiber diet (25 g of fiber) and fluid supplementation (up to 1.5–2.0 L/day) and may improve stool frequency and decrease the need for laxatives.
- There is no evidence that dietary and lifestyle measures have any effect on constipation in the elderly; fiber supplements and simple osmotic laxatives are usually an adequate approach for constipation in these patients.
- In patients with colonic dilation, fiber supplementation should be avoided.
- Psyllium supplements and lactulose may be appropriate for the treatment of chronic constipation.
4.4 Medication
- Evacuation disorders respond poorly to standard oral laxative programs. If an evacuation disorder plays a considerable role in constipation, biofeedback and pelvic muscle training may be considered. Critical success factors are the patient’s level of motivation, the frequency of the training program, and participation of a behavioral psychologist and dietitian.
- If a dietary approach fails, polyethylene glycol (17 g PEG laxative for 14 days) or lubiprostone (24 mg twice per day) can be used to promote bowel function in patients with chronic constipation.
- Prokinetic agents (e.g., the 5-HT4 receptor agonist prucalopride) can be used in constipation-predominant IBS.
- Simple laxative agents, such as milk of magnesia, senna, bisacodyl, and stool softeners are a reasonable choice for treating constipation.
4.5 Surgery
- If there is persistent treatment failure in slow-transit constipation, then carefully selected, well evaluated and informed patients may benefit from total colectomy with ileorectal anastomosis. The exceptional indication for colectomy must be established in a specialized and experienced tertiary center. Disappointing results may be seen, with fecal incontinence due to surgery and recurrent constipation, especially in patients with evacuation disorder.
- Only very few patients benefit from a (reversible) colostomy to treat constipation.
4.6 Evidence-based summary
Table 9 Summary: evidence base for the treatment of constipation (adapted from Rao SS, Gastrointest Endosc Clin N Am 2009;19:117–39)
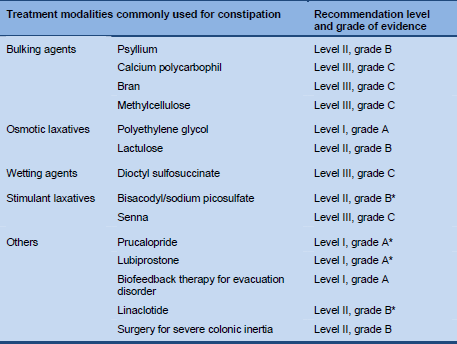
* Adapted by the present constipation guideline review team.
4.7 Cascade options for treatment of chronic constipation
The following cascade is intended for patients with chronic constipation without alarm symptoms and with little or no suspicion of an evacuation disorder. The main symptoms would be hard stools and/or infrequent bowel movements.
Level 1—limited resources
- Dietary advice (fiber and fluid)
- Fiber supplementation
- Milk of magnesia (magnesium hydroxide in an aqueous solution)
- Stimulant laxatives (bisacodyl better than senna) for temporary use
Level 2—medium resources
- Dietary advice (fiber and fluid)
- Fiber supplementation, psyllium
- Milk of magnesia, lactulose, macrogol
- Stimulant laxatives for temporary use
Level 3—extensive resources
- Dietary advice (fiber and fluid)
- Psyllium or lactulose
- Macrogol or lubiprostone
- Prokinetics (prucalopride)
- Stimulant laxatives (bisacodyl or sodium picosulfate)
4.8 Cascade options for treatment of evacuation disorders
This cascade is for patients with chronic constipation without alarm symptoms, but with suspicion of an evacuation disorder. The main symptoms would be prolonged straining, a feeling of incomplete evacuation, thin stools, a feeling of blockage, or failure of treatment for constipation with hard stools.
Level 1—limited resources
- Dietary and behavioral advice (fiber, fluid, timed bowel training)
- Therapy for chronic constipation
Level 2—medium resources
- Dietary and behavioral advice (fiber, fluid, timed bowel training)
- Therapy for chronic constipation
- Biofeedback therapy
Level 3—extensive resources
- Dietary and behavioral advice (fiber, fluid, timed bowel training)
- Therapy for chronic constipation
- Biofeedback therapy
- Surgical evaluation














