1. WGO global perspective—resource-sensitive guidelines and “cascades"
This guideline will be of interest to all health professionals in primary and secondary care involved in the management of people with hepatitis C infection in different countries of the world. It covers all stages of the disease management pathway: screening, testing, diagnosis, referral, treatment, care, and follow-up of children and adults with, or exposed to, hepatitis C (HCV) infection.
Numerous guidelines produced annually by prestigious medical bodies outline “gold standard” practices and are aimed at physicians in resource-rich environments. The main international guidelines on the management of hepatitis C are listed in Table 1.
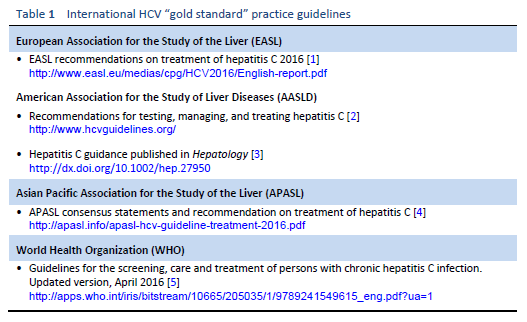
Some of these guidelines may therefore be inaccessible and irrelevant for many clinicians in developing countries. Any Western guidelines that fail to acknowledge this may be preventing the dissemination of knowledge and evidence to the full global audience. The WGO has developed the concept of “cascades” in order to make guidelines more applicable to differing resource environments, by providing a collection of related diagnostic and treatment options arranged hierarchically in terms of conditions and available resources [6].
WGO guidelines include alternatives for clinicians with limited funding. These alternatives are usually suggested on the basis of cost, but may also take account of local availability, technology, and infrastructure. Cascades can range from a simple list of options to more complex parallel diagnostic and treatment pathways, and they are transformed from being “resource-blind” to “resource-sensitive.” Inevitably, cascades are more heavily based on empirical evidence, rather than gold standard options. Research funding is usually spent on trying to improve “best practice” rather than the practicalities of delivery in developing countries. However, with strong involvement from experienced clinicians in developing countries, a consensus is usually reached. More widespread use of cascades in guidelines may also motivate research into the best options for resource-limited services.
2. Epidemiology—global comparison and resource factors
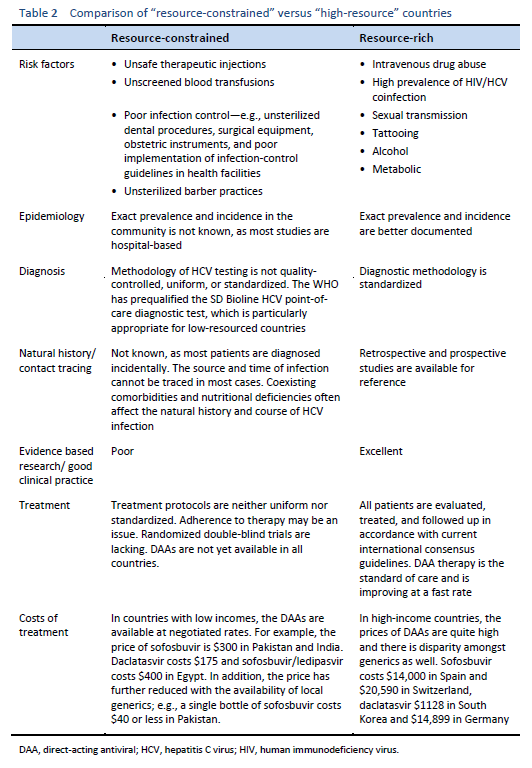
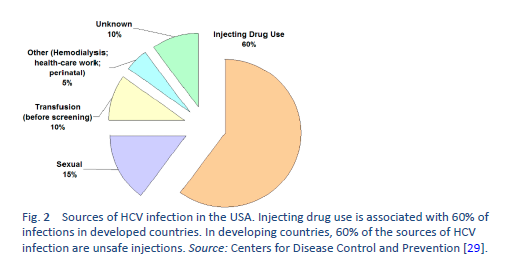
When the epidemiology of HCV infection globally is being discussed, it is imperative to discuss “north–west” and “east–south” differences as well. These include a low prevalence of HCV infection in the “north” and “west” [7,8] and a moderate to high prevalence [9–11] in the “south” and “east”—leading to a high health-resource and financial burden on already resource-constrained countries. The main risk factor for HCV in the “east” is unsafe therapeutic injections, due to poor practical application of universal infection control guidelines, including reutilization of syringes, needles, or any equipment from patient to patient without adequate sterilization techniques. This affects the treatment strategies in developing countries and emphasizes the need for prevention strategies, public awareness, health education, and sensitizing health-care staff and concerned authorities in governments. On the other hand, in Western developed countries, HCV is mainly transmitted by injecting drug users sharing injection equipment. The prevalence of anti-HCV among intravenous drug users may range from 35% to 61% [12,13], and intravenous drug use accounts for 60–80% of new HCV infections in the United States.
Another factor is the availability, cost, and quality of diagnostic tests for HCV infection, which make screening extremely difficult even in high-risk populations, leading to inaccurate data collection and reporting. Similarly, the standardization and methodology of polymerase chain reaction (PCR) testing makes deciding “whom to treat” even more difficult.
The natural history of HCV is also different in the “east” and “west,” due to specific risk factors such as alcohol use, addiction, intravenous drug use, co-infections, and superinfections. Other comorbidities (such as the prevalence of obesity and the metabolic syndrome) and nutritional deficiencies also affect liver histology and progression of the disease.
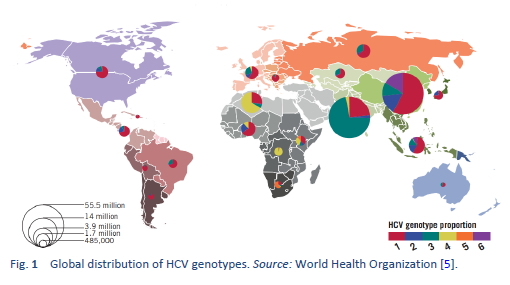
Hepatitis C is a contagious liver disease caused by the hepatitis C virus (HCV). The virus is endemic throughout the world, and a recent analysis including 1217 studies representing 117 countries and 90% of the global population concluded that approximately 180 million people worldwide are HCV-seropositive. It was calculated that HCV genotype 1 is the most prevalent worldwide, comprising 83.4 million cases (46.2% of all HCV cases), approximately one-third of which are in East Asia. Genotype 3 is the next most prevalent globally (54.3 million, 30.1%); genotypes 2, 4, and 6 are responsible for a total 22.8% of all cases; genotype 5 makes up the remaining < 1%.
Intravenous drug use, tattooing, and medical procedures such as dialysis and blood transfusion before the era of HCV screening have all contributed to the wide spread of HCV. It has become a major recognized health problem worldwide [14]. The distribution of HCV infection shows considerable geographic variation, with a higher prevalence in countries in east Asia, Latin America, the Mediterranean, and certain areas in Africa and eastern Europe.
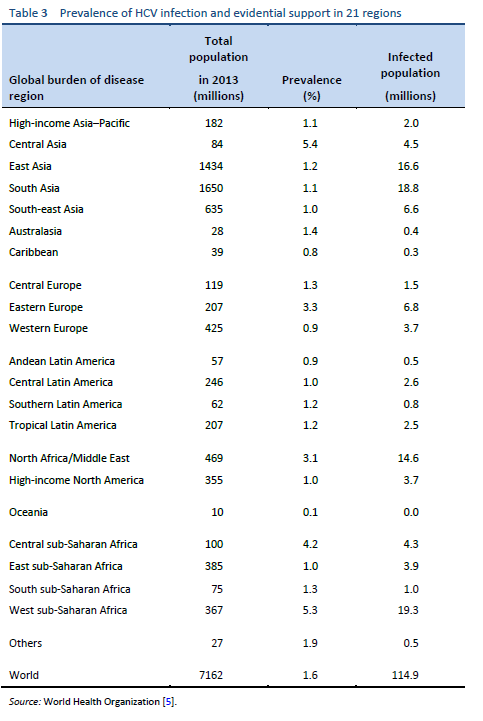
Hepatitis C is a global challenge. The data on the global prevalence are mostly based on HCV seroprevalence studies [15]. However, WHO data are based on published studies and data submitted from different countries and regions. Although HCV is a world epidemic, there is great variability in its distribution in different regions of the world [5].
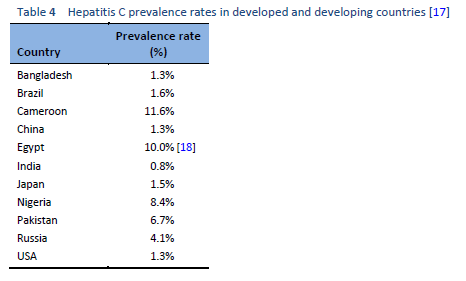
The highest prevalence rates are reported from developing poor countries in Africa and Asia, while the developed, industrialized nations in Europe and North America have low prevalence rates. Countries with high rates of chronic infection are China, Pakistan, Nigeria, and Egypt. Unfortunately, there are no good data from African countries, with the exception of Egypt, Morocco, and South Africa. The major transmission route in these countries is thought to be unsafe injections using contaminated equipment—as in the case of Egypt, where the HCV epidemic has been mainly attributed to the prolonged use of parenteral antischistosomal treatment (antimony potassium tartrate, tartar emetics) with use of nondisposable glass syringes for more than 30 years.
Chronic hepatitis C is the most common cause of cirrhosis and the most common indication for liver transplantation in Europe, North and South America, Australia, Japan, and Egypt. Patients’ risk of developing cirrhosis ranges from 5% to 25% over periods of 25–30 years [16].
HCV is transmitted primarily through percutaneous exposure: injecting drug use, needlestick injuries, and inadequate infection control in health-care settings; nosocomial infections are still occurring throughout the world. Less frequently, HCV transmission occurs among human immunodeficiency virus (HIV)-positive men who have sex with men (MSM) as a result of sexual contact with an HIV-infected partner [19], and among infants born to HCV-infected mothers. Overuse of injections and unsafe injection practices cause an estimated 2–5 million HCV infections globally [16,20–27].
The risk of HCV transmission in a monogamous heterosexual relationship appears to be very low (1.2% or lower) and the maximum incidence of HCV transmission through sex was 0.07% per year or approximately one per 190,000 sexual contacts [28].
3. Natural history and prevention
3.1 Natural history
Infections can range in severity from a mild illness lasting only a few weeks to serious illness (acute infection) or lifelong illness (chronic infection). Approximately 80% of patients infected with HCV will become chronically infected, and most of these patients will show evidence of chronic hepatitis. The incubation period is 14–180 days (average 45 days), and no vaccine against hepatitis C is currently available.
Predictors of chronicity in HCV infection [30]:
- Male sex
- Age > 25 years at moment of infection
- Acute infection is asymptomatic
- African-American ethnicity
- HIV infection
- Immunosuppression
Hepatitis C infection is usually slowly progressive over a period of many years, and between 5% and 15% of patients with chronic hepatitis may progress to developing liver cirrhosis over a period of 20 years [31]. However, several studies have suggested a more benign course of the disease [32] and indicate that fibrosis is a highly unpredictable process [33]. Approximately 80% of patients infected with HCV will become chronically infected, although some studies suggest higher rates of spontaneous clearance, particularly in young people [34,35].
Approximately 4–9% of patients with cirrhosis annually will develop progressive liver failure (decompensation), with a 1–4% annual risk of developing primary hepatocellular carcinoma (HCC) [36]. The mortality rate due to progressive liver failure or HCC will continue to increase during the next few decades. In some countries, HCV infection is the main cause of death from liver disease and is the leading indication for liver transplantation [37].
Approximately 70–80% of patients with hepatitis C are asymptomatic; in acute or acute-on-chronic hepatitis, the symptoms of all types viral hepatitis are similar and can include one or more of the following: fatigue, abdominal pain, poor appetite, jaundice.
3.2 Prevention
3.2.1 Counseling of infected persons on avoiding transmission of HCV
- The infected person should cover any bleeding wound or cuts and apply disinfectants immediately in order to keep the blood away from others..
- An infected person should not donate blood or organs, although organs from HCV-positive patients may be used in those who are already HCV-positive.
- Those who inject drugs should be counseled regarding the risk of transmission of HCV and advised to inject safely if they intend to continue.
- Vomit and other bodily secretions from an HCV-infected patient should be disposed of with disinfectant—e.g., bleaching powder and glutaraldehyde solution.
- The risk of sexual transmission of HCV is low. Spouses are not recommended to take barrier precautions as a risk reduction strategy. However, HCV infection in non-IDU men who have sex with men (MSM) has increasingly been reported in the literature, especially in HIV-positive patients.
- Transmission of HCV is low through breast milk, so breast-feeding should not be stopped.
- Household contacts and physical contact are not recognized risk factors for HCV transmission, so an HCV-infected person should not be barred from any activities of normal life.
3.2.2 Prevention in the community and health-care settings
- All blood donors must be screened for hepatitis C antibodies and/or HCV RNA.
- In health-care settings, adherence to universal precautions for infection control is essential. This should include the use of disposable or adequately sterilized materials for invasive procedures, and adequate cleansing and sterilization of instruments.
- It is important to educate tattooists, barbers, foot/hand care workers, and practitioners of traditional or alternative therapies about ways of minimizing blood contamination. This involves sterilization techniques for procedures that involve skin penetration or breaks to mucosal surfaces.
- As transmission of HCV via injecting drug use is an increasing trend, it is important to implement an education campaign about the harm of drug use, especially among school-age children. Harm reduction programs such as needle/syringe programs should also be implemented.
- Those who have received surgical or dental treatment can be at risk of acquiring HCV infection and should be offered testing, especially in health-care systems in which transmission associated with surgical and dental treatment has been a major risk.
- Individuals with a history of blood transfusion have a higher risk of HCV infection and should be offered testing.
- Chronic hepatitis C patients should be vaccinated against hepatitis B after screening.
- Use of injections is only preferable in approved health-care settings, and injections should be discouraged as much as possible, especially in settings in which medical supervision is not available. When mandatory, injections must be carried out in accordance with WHO recommendations on safe injecting practice.
- An appropriate protocol for needlestick injury should be drawn up and followed in all hospitals (public and private), as recommended by the Centers for Disease Control and Prevention (CDC).
- All skin lesions on the hands of health-care workers should be covered with waterproof dressing, and if possible double gloving with a blood indicator in the glove should be used.
- Health-care workers should be vaccinated for HBV.
4. Diagnosis and screening
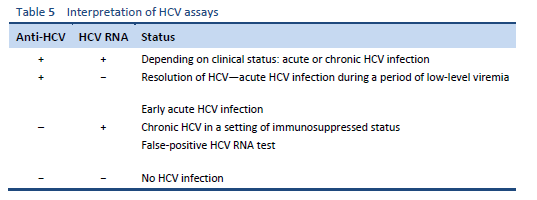
Infection with HCV is diagnosed by testing for specific antibodies using enzyme-linked immunoassay (ELISA). The presence of HCV antibody shows that a person has been infected with HCV virus, but it does not indicate whether the infection is acute or chronic, or has resolved. Antibodies may not be detectable during the first few weeks after initial infection, due to the “window period,” or if the patient is immunocompromised.
In contrast to hepatitis A and hepatitis B virus, in which the diagnosis of acute infection is based on immunoglobulin M (IgM) antibodies, there is no serologic marker for acute HCV infection. Screening tests for chronic HCV infection are enzyme immunoassay (EIA) or chemiluminescence immunoassay (CIA) for anti-HCV and verification by an additional, more specific assay—e.g., nucleic acid testing for HCV RNA.
Diagnosis of acute hepatitis C is based on:
- With or without marked elevation of alanine aminotransferase (ALT is sometimes raised by more than 10 ×)
- With or without jaundice
- Detectable serum HCV RNA
- Followed by anti-HCV seroconversion weeks later
If both anti-HCV and HCV RNA are detectable from the start, differential diagnosis between acute and chronic HCV infection with a flare of ALT may be difficult.
The WHO has prequalified the first rapid HCV diagnostic test, a tool that will aid diagnosis of HCV in low-income and middle-income countries and improve access to treatment. The newly prequalified test, SD Bioline HCV, manufactured by Standard Diagnostics, Inc. (South Korea), is a point-of-care diagnostic method, which makes it particularly appropriate for low-resourced countries in which testing laboratories and trained personnel may be scarce. Resembling a pharmacy pregnancy test, it does not require hospital facilities or electricity and can be performed by health workers with limited training. The test gives a result within 20 minutes. The product has not been validated for infants or children [38].
Screening is recommended for at-risk groups and specific age groups—risk factors vary from country to country, as does the HCV infection risk in various groups. If screening according to the list in Table 6 is not practicable (for example, are patients aware that they have been injected with used syringes?), patients should be screened on the basis of elevated aminotransferase levels at their initial presentation. The Centers for Disease Control in the USA recommend testing all baby boomers; in Europe, this is not recommended.

5. Management of HCV infection
5.1 Treatment goals
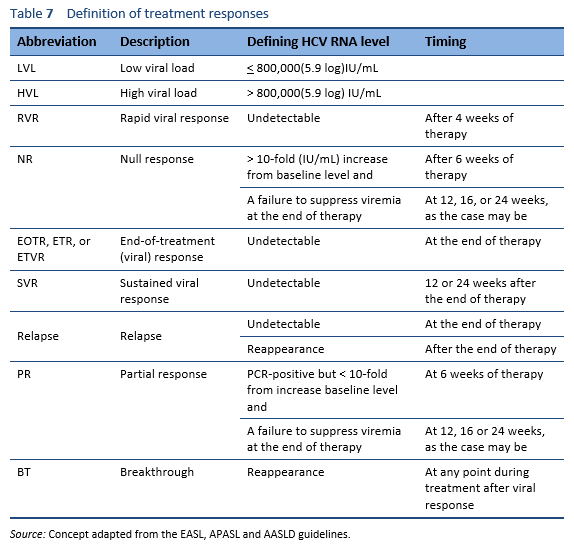
The goal in treating HCV infection is to reduce virus-related complications. This goal is achieved by eradicating the virus to achieve a sustained viral response (SVR). Patients who achieve SVR have clearance of the virus, and the chances of virus reactivation are negligible. Improvements in liver necroinflammation, fibrosis, and in the risk of hepatocellular carcinoma have been demonstrated in patients who have achieved an SVR.
Patients in whom an acute HCV infection has resolved without therapy do not require antiviral treatment. Depending on the sources, between 15% and 50% of patients are reported to recover spontaneously.
5.2 Treatment principles
Treatment and cure of hepatitis C has been shown to prevent the long-term risk of complications and is the primary form of management for chronic HCV infection.
Non-1 HCV genotypes are the most common in densely populated countries of South Asia, East Asia, Africa, and the Middle East. Before the era of pegylated interferon (PEG-IFN), conventional IFN monotherapy or conventional interferon/ribavirin (IFN/RBV) combination therapy was the mainstay of HCV treatment in most parts of the world. After 2002, PEG-IFN/RBV became available. The pivotal clinical trials on PEG-IFN/RBV therapy showed an SVR of 40–45% in patients with genotype 1, up to 80% in those with genotype 2, and only 50% in those with genotype 3a. All of these treatments left about 50–60% of chronic hepatitis C patients as either nonresponders or relapsers [35]. In addition, these therapies required 24–48 weeks of injections with interferon and ribavirin, with significant toxicity, and too many of the patients were ineligible for IFN or were reluctant to accept the treatment due to adverse effects. An ideal regimen was therefore required that would involve all-oral drugs, one daily dosage, a short duration of therapy, and minimal side effects and would be pan-genotype and have a high SVR value (> 95%), regardless of the stage of liver fibrosis, prior nonresponse to IFN/RBV treatment, gender, race, and age [39].
After 2011, this led to the era of direct-acting antivirals (DAA), which are the current standard of care.
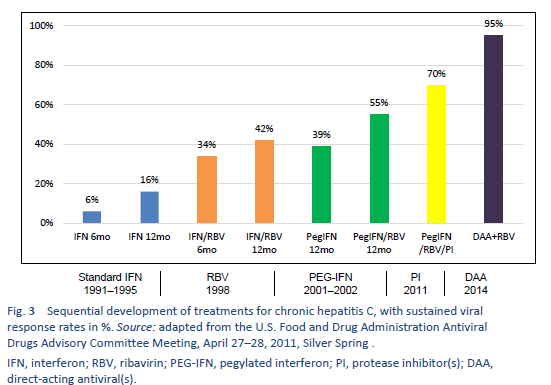
Sofosbuvir (SOF), which is easily available in the developing world, is taken as a 400-mg tablet once daily. PEG-IFN-a2a should be used at a dosage of 180 µg once per week, whereas PEG-IFN alfa-2b should be used at a weight-based dosage of 1.5 µg/kg per week. The ribavirin (RBV) dosage depends on the patient’s weight (e.g., 1000 mg < 75 kg and 1200 mg > 75 kg.)
The new DAAs were initially available only at a very high price. Efforts were made to negotiate the prices for low-income countries. This led to diversity in prices in different regions around the globe. For example, a single pack of sofosbuvir was launched in the United States at a price of $28,000 with a 3-month coarse costing around $84,000 while the same generic is available in Pakistan and India at a price of $300 (a complete course of 6 months costs $1800).
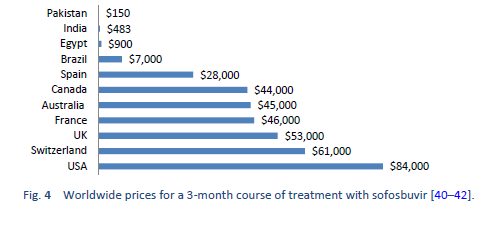
Gilead Sciences, Inc., the manufacturer of Sofosbuvir, has signed voluntary licensing agreements with several manufacturers to market local versions of these generic medicines in some low-income and middle-income countries, as a result of which the prices have further declined in those specific countries. However, these voluntary licensing agreements do not apply in several middle-income countries that also have a major disease burden, such as China, Brazil, Argentina, Iran, and Turkey. The generic versions of the DAAs are a quite cheap and effective alternative. In a study of 448 patients, Freeman et al. reported an SVR4 of approximately 94% using generic DAAs [43].
The treatment options discussed in the guidelines should be selected on the basis of the health-care and financial resources available. All options are based on empirical evidence from regional experts and societies, with little divergence from evidence-based medical practice. This represents an effort to make hepatitis C treatment globally applicable and practically feasible, updating the information available to practicing physicians at all levels and thus allowing hepatitis C patients all over the world to receive maximum benefit.
Finally, the guidelines are not fixed rules, but represent a dynamic and ever-changing process, guiding physicians to treat patients accordingly and taking into account individualized approaches wherever needed. The guidelines always need improvement and updating as newer studies and evidence become available.
5.3 Who should be treated?
HCV infection can be cured by antiviral treatment; however, due to the asymptomatic nature of the disease, many infected individuals are unaware of the infection, and for those who are diagnosed, access to treatment remains poor in many settings [5].
An “advocacy brief” published by the World Health Organization sets out the target of eliminating hepatitis B and C by 2030 [44]. All patients with chronic HCV infection should therefore receive therapy, except for patients with a short life expectancy due to severe comorbid conditions. Patients who are at high risk for liver-related complications should be prioritized for immediate treatment.
Patients who are at high risk for liver related complications include the following:
- Patients with advanced fibrosis with metavir stages ≥ F2
- Patients with decompensated cirrhosis who are waiting for liver transplantation
- Patients with liver transplants
- Patients with severe extrahepatic complications such as vasculitis, cryoglobulinemia causing end organ damage, or glomerulonephritis/nephrotic syndrome causing significant proteinuria
5.4 Treatment response predictors
Recommendations for preferred HCV treatment regimens are continuing to evolve, but they still depend on several factors that compromise a SVR in some cases:
- HCV genotype
- Prior HCV treatment history
- Compensated versus decompensated liver disease
- Drug–drug interactions
- Chronic kidney disease
- Solid organ transplant recipients
The following are predictive factors for a less favorable response to treatment with DAA combinations:
- Previous treatment with DAAs
- Liver cirrhosis (Child–Pugh B and C)
- Poor treatment adherence
5.5 Pretreatment assessment
The following should be assessed before DAA treatment is started:
- Taking a detailed history and physical examination is essential, including the patient’s history of any other liver diseases and medical conditions that can adversely affect liver status, such as hepatitis B infection, alcoholism, autoimmunity, metabolic liver diseases, or hepatotoxic drugs. These should be inquired into and appropriate measures should be taken to reduce the risks.
- Other evaluations should be considered (cardiopulmonary and psychiatric evaluation) and the risk of nonadherence should be assessed. Appropriate measures should be taken to reduce these risks.
- Assessment for current/prior medications and adherence to previous treatments. Significant potential drug–drug interactions should also be assessed.
- The extent of hepatic fibrosis should be checked using noninvasive measures:
— Studies have demonstrated that FibroScan is a sensitive alternative to liver biopsy. The amount of fibrosis can be quantified very easily and reliably in more than 95% of the patients [45]. A correct interpretation of transient elastography must have an interquartile range/median values of < 30% and serum ALT < 5 × upper limit of normal. There should be no ongoing excessive alcohol intake, and the patient’s BMI should be taken into account. If the BMI is over 30 kg/m2, using extralarge (XL) probes may be considered.
— In resource-limited regions and in places where FibroScan is not readily available, scores such as the fibrosis 4 index (FIB4), AST to platelet ratio index (APRI), and acoustic radiation force impulse (ARFI) can be used. An APRI score ≥ 2 can be used to predict the presence of cirrhosis. At its cut-off point, the ARFI score has a sensitivity of 48% but a specificity of 94% for predicting cirrhosis. It can also be used to predict the presence of significant fibrosis (stages 2–4). Using a cut-off value of 1.5, the sensitivity is 37% and the specificity is 95% for significant fibrosis [46,47].
— Liver biopsy can only be considered when there is possibility of additional etiology.
- Serum quantitation of HCV-RNA using a sensitive real-time PCR-based assay with a lower limit of detection of ≤ 15 IU/mL.
- HCV genotyping.
- In patients with suspected or known cirrhosis, the Child–Turcotte–Pugh score and Model for End-Stage Liver Disease (MELD) score should be calculated.
5.5.1 Recommendations for pretreatment monitoring
- A complete blood count(CBC), liver function tests (LFTs), serum albumin, INR, glomerular filtration rate (GFR), and a thyroid-stimulating hormone (TSH) test (if an IFN regimen is planned) should be performed within 12 weeks of the start of therapy.
- Quantitative PCR and genotyping anytime should be done before the start of therapy.
- Women of childbearing age must undergo urinary pregnancy testing before the start of therapy. Currently, all new oral therapy regimens, with or without ribavirin, are contraindicated for use during pregnancy or during breastfeeding due to a lack of sufficient safety information in humans, and adequate measures of contraception are still routinely recommended for female patients of childbearing age [48].
- With ribavirin-containing regimens, the patient’s age and cardiovascular comorbidity due to secondary anemia should be taken into account.
5.6 On-treatment assessment
Recommendations for patients receiving HCV treatment during their therapy:
- Compliance should be encouraged either at clinical visits or by phone. Any adverse events should be inquired about, and advice regarding drug–drug interactions should be given. The University of Liverpool provides a “HEP Drug Interaction Checker” (http://www.hep-druginteractions.org) [49].
- CBC, serum creatinine, GFR, and LFT assessments should be performed after 4 weeks of treatment. CBCs can be done more often in patients receiving ribavirin, if clinically indicated.
- TSH tests should be done at 12 weeks for those patients receiving interferon.
- Quantitative PCR assessment should be performed at the end of treatment and then 12 weeks afterward.
5.6.1 When to stop the treatment because of side effects
- If there is a 10-fold or greater increase in ALT after 4 weeks of therapy.
- If there is a less than 10-fold increase in ALT with one of the following:
— Symptoms developing (nausea, vomiting, weakness)
— Jaundice
—Increases in bilirubin, alkaline phosphatase (ALP), or INR
- If there is a less than 10-fold increase in ALT and the patient is asymptomatic, ALT assessment should be repeated at 6 weeks; if it is persistently high, one can consider stopping therapy.
5.7 Post-treatment assessment
5.7.1 For patients who fail to respond to treatment
- LFTs, CBC, INR every 6–12 months to assess disease progression (F4 patients).
- HCC surveillance for patients with advanced fibrosis (metavir F3/F4) using ultrasonography every 6 months.
- Endoscopic surveillance for varices in cirrhotic patients.
- Re-treatment evaluation once an effective alternative treatment is available. Pretreatment mutation testing is needed to select the best regimen at present. Currently, the guidelines recommend NS5A resistance evaluation in patients in whom DAAs fail, especially before considering elbasvir/grazoprevir for genotype 1a patients.
5.7.2 For patients who achieve an SVR
- For patients with F0–F2 fibrosis, the same recommendations apply as if they were never infected with HCV.
- For patients with F3–F4 fibrosis, twice-yearly ultrasound monitoring is recommended for HCC surveillance.
- Baseline endoscopic surveillance for cirrhotic patients, and if varices are found they should be treated and followed up in the standard way.
- If LFTs are persistently abnormal despite an SVR, other causes of liver disease should be assessed.
5.8 Interferon-free all-oral treatment regimens for CHC since 2014
- In December 2013 and January 2014, second-generation DAAs were approved by the FDA for treatment of chronic hepatitis genotype 1, which is the most prevalent genotype and one that is considered to be difficult to treat. The first of these was sofosbuvir (SOF), which is an NS5B polymerase inhibitor. The first trial using SOF/RBV was the ELECTRON study. This reported SVRs in 84% of 25 treatment-naïve patients after 24 weeks of therapy (SVR 24) [50,51]. In a subsequent study of a regimen consisting of sofosbuvir and RBV for 24 weeks in 60 treatment-naïve patients with genotype 1 with poor prognostic factors such as African-American ethnicity, ccIL28B, and viral load more than 800,000 IU/mL, the SVR 24 rates were 68% [51].
- In the ELECTRON study, SOF/RBV was administered in genotype 2 and 3 patients without cirrhosis, and 100% achieved SVR 24 [52]. In the FISSION trial, with SOF/RBV administered for 12 weeks in 499 treatment-naïve patients, the SVR 12 rates were 97% for patients with HCV genotype 2. In genotype 3 patients, the SVR 12 rates were only 56% [53]. Similarly, in the POSITRON trial the SVR 24 rates after 24 weeks of treatment were 61% for genotype 3 and 93% for genotype 2 [54]. Similar results were achieved in the FUSSION trial, with poor SVR rates of 62% for patients infected with genotype 3.
- Since then, a number of drugs in separate or fixed-dose combinations have been approved by the FDA, including daclatasvir, 3D Regimen, paritaprevir + dasabuvir/ombitasvir + ritonavir (Viekira pak).
- One of the most recent FDA-approved pan-genotype combinations, available under the trade name Epclusa, contains sofosbuvir + velpatasvir [55]. In a phase 3 trial, Feld et al. administered this combination for 12 weeks and reported an SVR 12 of 99% in patients with genotypes 1, 2, 4, 5, and 6 [56]. The ASTRAL-3 trial reported an SVR 12 of 95% in genotype 3 patients treated with sofosbuvir + velpatasvir for 12 weeks [57]. In another trial, Curry et al. used this combination in decompensated patients, with SVRs of 83% in patients who used the combination for 12 weeks, 86% in those who used it for 24 weeks, and 94% in patients who used the combination along with ribavirin for 12 weeks [58].
- Zepatier is another pan-genotypic DAA-based combination approved in 2016. The combination contains 100 mg of grazoprevir and 50 mg of elbasvir. A combination of Zepatier with sofosbuvir was investigated in the C-SWIFT study, with an efficacy of more than 90% when given for 8 weeks in patients with genotype 1 and 12 weeks in those with genotype 3 [59].
5.9 Treatment of special populations
5.9.1 Treatment of acute hepatitis C
Acute hepatitis C is difficult to diagnose in asymptomatic patients, particularly when the exact time of acquisition of the virus is not definite. Two issues need to be addressed in acute hepatitis C patients: firstly, when to start the therapy; and secondly, what the regimen and duration of the therapy should be.
In one meta-analysis of 16 studies, the outcome in the group of patients who were offered early therapy in acute hepatitis was better than that in the group of patients who were observed for spontaneous clearance. In another study, early therapy with higher doses of conventional IFN achieved an SVR of 85–100%. The dose of conventional IFN was 5–10 million units/day for 12 weeks. PEG-IFN at a dosage of 1.2–1.3 mg/kg weekly was another choice, in view of the convenient dosage schedule, but it had a higher cost [60–65].
A study by Deterding et al. showed that delayed treatment is as effective as immediate treatment. In addition, delayed treatment can reduce the possibility of unnecessary treatment in patients who are able to clear the virus spontaneously without any treatment; however, close monitoring is required in these cases [66].
As the new DAAs have better efficacy and safety, the argument for early treatment has become relatively weaker. The new recommendations are therefore as follows.
- Regular laboratory monitoring with HCV RNA is recommended at least for 6 months in order to assess spontaneous clearance.
- Counseling is required for patients with acute HCV infection to ensure avoidance hepatotoxic drugs (e.g., acetaminophen) and alcohol. The patients should also take precautionary measures to reduce the risk of transmitting the disease to others.
- Early treatment can only be considered in special circumstances—e.g., in individuals who are at risk of transmitting the disease to others (such as intravenous drug abusers, or surgeons), patients who are already suffering from advanced liver disease for some other reason, and those in whom the chances of being lost to follow-up are greater. Even in these patients, one should at least wait for 12–16 weeks before starting therapy.
- If indicated, treatment for acute HCV infection can be administered using DAAs with the same regimens as for chronic disease.
- Prophylactic therapy is not recommended in needlestick injuries, as the infectivity rate is very low.
5.9.2 Treatment of HCV infection in children
There are different schools of thought regarding treatment for hepatitis C in children. As the natural course of chronic hepatitis C infection is slow, treatment can be deferred until adolescence. However, the adolescent and young adult age group is also thought to be more carefree and less likely to be compliant with treatment.
The AASLD still recommends only interferons along with RBV in the pediatric population, and PEG-IFN is thought to be superior to conventional IFN in children as well [67]. Peginterferon alfa-2b is administered at a dosage of 60 µg/m2/week, whereas peginterferon alfa-2a is given at a dosage of 180 µg/1.73 m2/week along with RBV at a dosage of 15 mg/kg/day. The combination is administered for 48 weeks in patients with genotypes 1 or 4, and for 24 weeks in those with for genotypes 2 or 3.
The pediatric response to IFN/RBV therapy in HCV infection is 36–57% SVR for genotype 1, 84–100% for genotypes 2 and 3, and 50–80% for genotype 4. The side effect profile of IFN/RBV in children includes flu-like symptoms, fever, leukopenia, headaches, abdominal pain, loss of appetite, diarrhea, and psychiatric effects [68–72].
Due to the side effects and low SVR, particularly in genotype 1 in children, the use of DAAs needs consideration. Two trials sponsored by Gilead Sciences are in phase 2, evaluating the efficacy and safety of ledipasvir and sofosbuvir for genotype 1 and sofosbuvir and RBV for genotypes 2 and 3, respectively. The excellent efficacy of the new DAAs in the adult population has encouraged researchers to evaluate the drugs in the adolescent population. The pharmacokinetics and safety profile of sofosbuvir and ledipasvir/sofosbuvir have been evaluated in children in the 12–17-year-old age group, and comparable results have been reported in this group [73]. However, more in-depth trials are required for approval of these DAAs in children.
Notes:
- Other special groups of CHC patients—e.g., patients with renal failure, those with coinfection with HCV/HIV and HBV, organ transplant recipients, and others are treated in specialized liver units; practice recommendations can be found in other international guidelines (see Table 1).
- As the treatment of hepatitis C is rapidly changing due to the rapid pace of development of DAAs, readers are referred to the AASLD, EASL, and APASL guidelines for new treatment algorithms.
5.10 Are local generics and proprietary DAAs effective?
With the approval of new drugs and new DAAs, expectations have arisen that these medications will provide effective, safe, and cheap treatment for HCV. Although there is dramatic diversity in the price of generics around the globe, Hill et al. pointed out in 2013 that the original manufacturing costs of the DAAs are very low—e.g., a 12-week treatment course of sofosbuvir costs approximately $101, and a 12-week course of daclatasvir costs $20 [74].
Several generic manufacturers are available currently, and the competition amongst them is leading to further reductions in the prices of DAAs. Low-income countries such as Pakistan and India have access to low-cost, active pharmaceutical ingredients, but the quality assurance of the products is questionable. In countries such as Egypt, where quality assurance is strictly applied by the legal authorities, the data on generic drugs are very promising. The WHO has developed a mechanism for quality assurance of these drugs through its prequalification program [75].
Preliminary data from one center in Pakistan, based on low-price generic sofosbuvir, showed a rapid viral response rate of 86.8% in genotype 3 patients (abstract submitted for Digestive Diseases Week 2017). Similarly, two abstracts presented in relation to generic treatment at the AASLD meeting in Boston, by groups from Russia and Qatar, have shown excellent results, with SVRs of approximately 92% and 95% respectively [76,77].
5.11 Is IFN still needed despite the advent of DAAs? A local perspective
Interferon has undoubtedly been the mainstream drug in the treatment of HCV in the past, but the large number of disadvantages with it and the introduction of safe and effective alternatives in the form of DAAs have limited its use, and IFN may become obsolete in the treatment of HCV in the near future. There are no medical reasons for withholding DAAs for HCV treatment, but in low-income countries—especially in the Asia–Pacific region—their penetration has so far been very slow. Currently, the only indication for IFN-based therapy is when there is no access to DAAs. Another argument in favor of IFN is the relatively better response to it in the Asian population, partly because of the IL28B allele; however, global viral eradication is not possible with IFN-based regimens. Access to DAAs is the main issue that needs to be addressed. The efforts being made by Gilead Sciences, Inc. in providing its medication to the 90 countries with the world’s lowest gross domestic product figures are an appreciable step in this regard.
5.12 Drug resistance to DAAs—an underestimated issue
HCV has a high replication rate, leading to a low proofreading capacity of HCV RNA–dependent RNA polymerase [78,79]. This leads to a high level of genetic variation even within a single genotype [80]. Thus, any individual infected with HCV has a mixture of genetically similar strains of HCV, with a predominant wild-type strain that is drug-sensitive (detectable at the start of therapy) and low levels of resistant strains (not detectable at the start of therapy). These resistant strains have a mutant amino acid that either makes the DAA less effective or makes the virus more fit [81].
As the treatment starts with DAAs, there is a rapid decline in the sensitive variant, making the HCV RNA quantitative analysis negative. If the duration of DAA therapy is long enough, then at the end of treatment the sensitive strains are cleared, leaving behind the resistant variant at low undetectable levels, so that the HCV RNA quantitative analyses are still negative. As the treatment stops, the change in the competitive environment (with elimination of the sensitive type) leads to the emergence of resistant strains, causing DAA relapse/failure.
Different resistance-associated strains are well documented for various DAAs, with variable prevalence. For example, common resistance-associated variants for sofosbuvir are L159F, V321A, and S282R. No data are available for the first two variants, whereas the third variant has low resistance, with a prevalence of about 0.4%. Daclatasvir has a high-resistance variant, M28, with a prevalence of 0.5–4.0%. Ledipasvir has two high-resistance variants, one of which has an up to 100% prevalence for genotypes 2 and 4 [82].
In general, resistance to NS34A protease inhibitors such as boceprevir disappears from the peripheral blood within a few weeks to months, whereas resistance to NS5A inhibitors—such as daclatasvir, ledipasvir, and ombitasvir—persists for years [81]. The NS5B nucleoside polymerase inhibitor sofosbuvir has the highest barrier to resistance [82].
DAA resistance is an emerging issue that requires further evaluation. Studies on pretreatment resistance are likely to be included in recommendations in the near future. Currently, the AASLD 2016 updated guidelines recommend NS5A resistance evaluation in patients in whom DAAs fail, particularly before elbasvir/grazoprevir are considered for genotype 1a patients [3]. Apart from genotype 1a, NS5A resistance is also very common in genotype 3, which is more prevalent in Pakistan. Greater insight is required in dealing with patients with DAA failure in Pakistan, and these patients—particularly when they have advanced fibrosis—may need reinforcement of therapy with sofosbuvir/velpatasvir [57,58]. HCV resistance to DAAs can prevent patients from achieving an SVR, and any patient whom DAAs fail should be managed in accordance with the recommendations made in the relevant section.
5.13 HCV treatment by non-specialist practitioners
With all oral DAAs, and particularly with the availability of pan-genotypic once-daily regimens, treatment for HCV appears to be very easy with limited assessment. As the prevalence of HCV is very high in several resource-limited countries and certified hepatologists and gastroenterologists are also scarce, flexibility is required in this regard. Kattakuzhy et al. initiated the ASCEND trial in order to determine the safety of HCV-related DAA therapy administered by nonspecialist practitioners, and it was found that this was equally safe and effective [83]. The results are encouraging, but bearing the local scenario in mind, some modifications and limitations should be implemented—such as ensuring proper training of general practitioners and encouraging them to follow the guidelines and carry out timely referral for complications and special populations. This can be successfully achieved using teleclinics—a model similar to the ECHO project [84].
5.14 Summary
Advantages of DAAs:
- All-oral therapy, no injection.
- Pan-genotypic: recent studies support the efficacy of DAAs in all genotypes (1 to 6).
- Absence of viral breakthrough during treatment and high barrier to resistance.
- Effective in IL28B CT, TT variants
- Simple dosage, no relation with foods, minimal side effects, and shorter duration of therapy.
- Effective in treatment-naïve and previously treated patients
- Safe and effective in cirrhotic patients.
Limitations of DAAs:
- The cost is high particularly for resource-constrained regions, and there are smaller published studies with little experience of usage in different groups of patients.
- Nonavailability in certain countries.
- Drug–drug interactions.
- DAA resistance.
6 Treatment categories and cascades
In this section, patients with chronic hepatitis C who are eligible for treatment in accordance with the diagnostic criteria are stratified following internationally accepted criteria into six categories based on genotype, whether treatment-naïve, failure of treatment, treatment response, and liver cirrhosis. These parameters are evidence-based and are an integral part of the AASLD, EASL, APASL, and other regional society treatment guidelines.
Several options are available to health-care providers, prioritizing on the basis of efficacy of therapy. Clinicians can select options on the basis of the patient’s liver status and drug availability/affordability.
Although the guidelines no longer recommend the use of interferon-based regimens, access to DAAs is still very difficult in several parts of the world, including developed and developing countries. There are even still a few countries in which the only treatment option for hepatitis C consists of interferons. Although interferon-based regimens are not mentioned as options in the cascades below, we would still recommend their use in regions in which oral drugs are not available. PEG-IFN alfa-2a should be used at a dosage of 180 µg once per week, whereas PEG-IFN alfa-2b should be used at a weight-based dosage of 1.5 µg/kg per week, as in the previous guidelines/cascades.
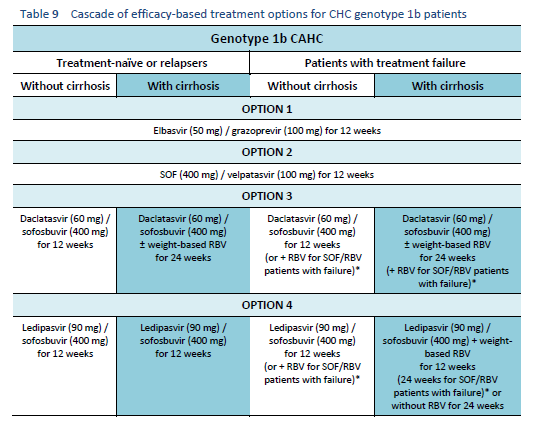
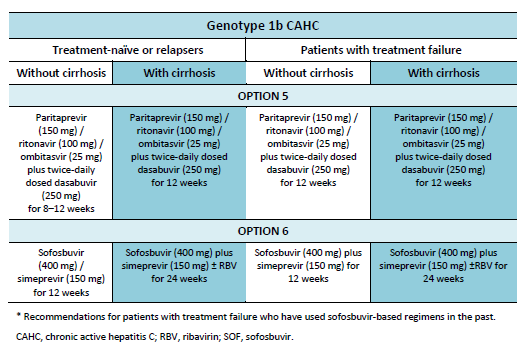
6.1 Cascades for CHC genotype 1
The following options are available for patients with chronic hepatitis C (CHC) genotype 1 infection, depending on their previous response and liver status.
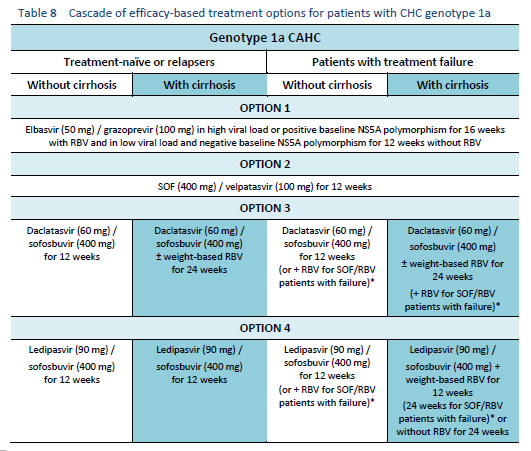
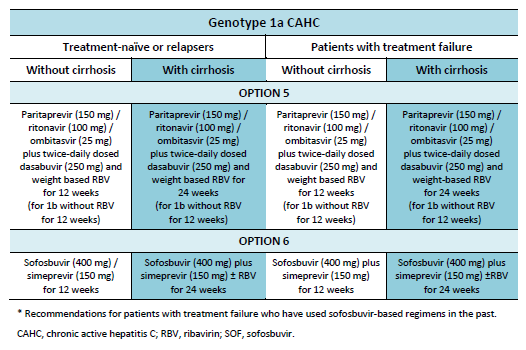
6.2 Cascades for CHC genotype 2
The following options are available for patients with chronic hepatitis C genotype 2 infection, depending on their previous response and liver status.
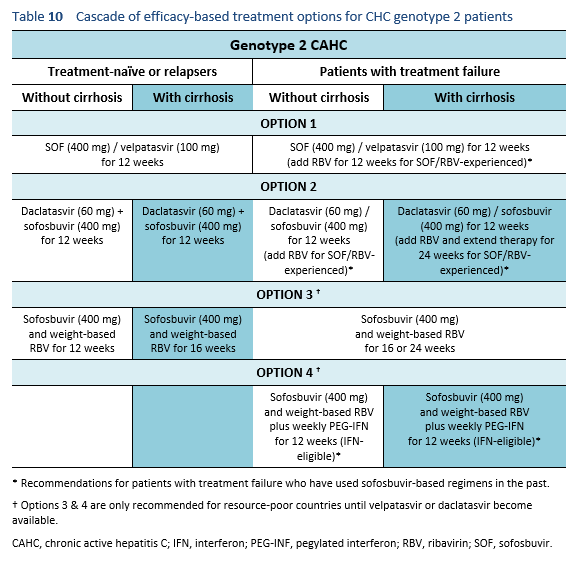
6.3 Cascades for CHC genotype 3
The following options are available for patients with chronic hepatitis C genotype 3 infection, depending on their previous response and liver status.
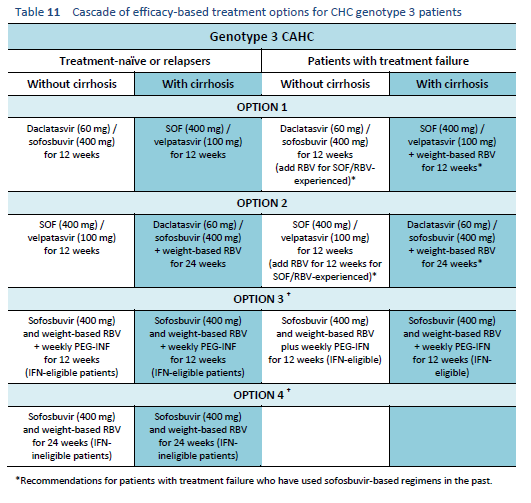

6.4 Cascades for CHC genotype 4
The following options are available for patients with chronic hepatitis C genotype 4 infection, depending on their previous response and liver status.
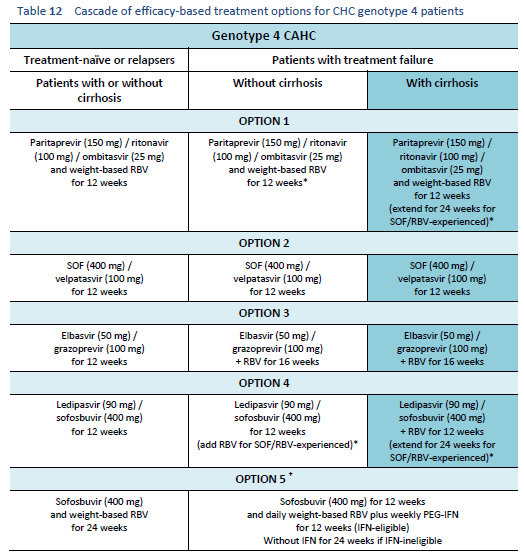

6.5 Cascades for CHC genotype 5/6
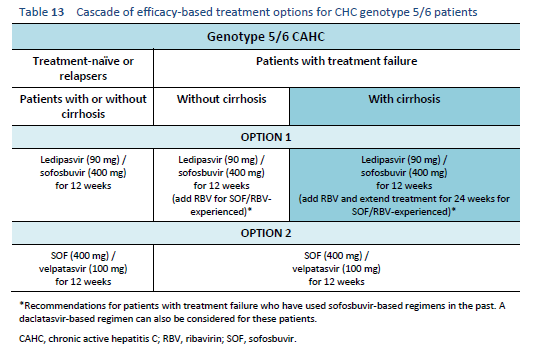
The following options are available for patients with chronic hepatitis C genotype 5 or 6 infection, depending on their previous response and liver status.
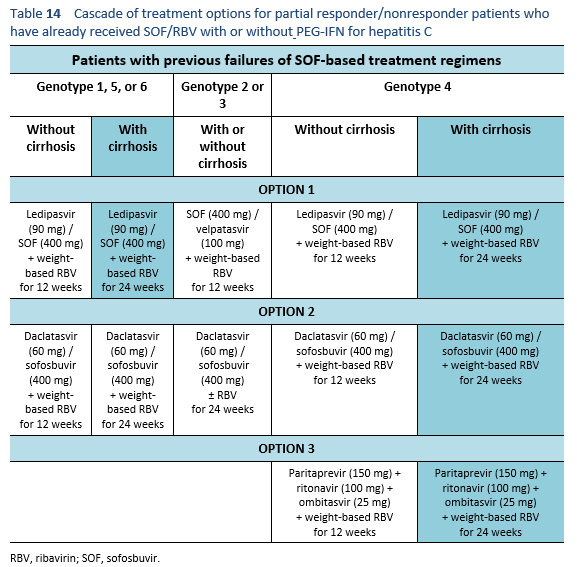
6.6 Cascades for treatment failure in patients with a sofosbuvir-based regimen
The following are options for patients who have already received SOF/RBV with or without PEG-IFN for hepatitis C, but were either partial responders or did not respond at all. Partial responders, by definition, are the patients whose viral load increases but < 1 log10 IU/mL in comparison with the baseline after 6 weeks of therapy.
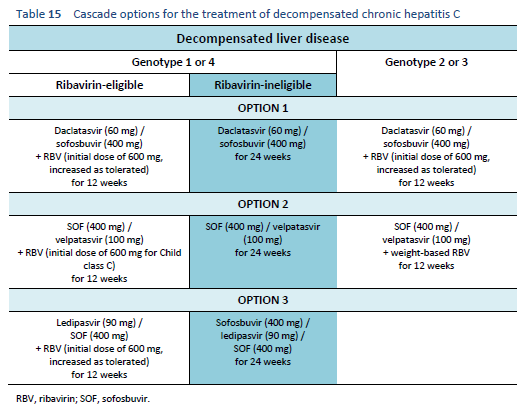
6.7 Cascades for decompensated chronic hepatitis C
6.8 Mixed genotypes or untypable genotypes
The data regarding the use of DAAs is very limited in patients with more than one genotype or with untypable genotypes. In these patients, it is best to wait until a pan-genotype drug becomes available. Until then, if treatment is inevitable, the choice, combination, and duration of DAAs should maximize efficacy against all represented genotypes, whereas genotype 1 regimens should be preferred for untypable cases.
7 References
1. European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2016. J Hepatol. 2017 Jan;66(1):153–94.
2. AASLD/IDSA HCV Guidance Panel. Recommendations for testing, managing, and treating hepatitis C [Internet]. American Association for the Study of Liver Diseases (AASLD); 2017 [cited 2017 Feb 5]. Available from: http://www.hcvguidelines.org/full-report-view.
3. AASLD/IDSA HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015 Sep;62(3):932–54.
4. Omata M, Kanda T, Wei L, Yu M-L, Chuang W-L, Ibrahim A, et al. APASL consensus statements and recommendation on treatment of hepatitis C. Hepatol Int. 2016 Sep;10(5):702–26.
5. World Health Organization. Guidelines for the screening, care and treatment of persons with chronic hepatitis C infection. Updated version, April 2016 [Internet]. Geneva: World Health Organization; 2016 [cited 2017 Jan 5]. 138 p. Available from: http://www.who.int/hepatitis/publications/hepatitis-c-guidelines-2016/en/.
6. Mandeville KL, Krabshuis J, Ladep NG, Mulder CJ, Quigley EM, Khan SA. Gastroenterology in developing countries: Issues and advances. World J Gastroenterol. 2009 Jun 21;15(23):2839–54.
7. Zou S, Tepper M, El Saadany S. Prediction of hepatitis C burden in Canada. Can J Gastroenterol J Can Gastroenterol. 2000 Aug;14(7):575–80.
8. Palitzsch KD, Hottenträger B, Schlottmann K, Frick E, Holstege A, Schölmerich J, et al. Prevalence of antibodies against hepatitis C virus in the adult German population. Eur J Gastroenterol Hepatol. 1999 Nov;11(11):1215–20.
9. Umar M, Khaar H-B, Khan AA, Mohsin A, Din W, Shah HA, et al. Diagnosis, management and prevention of hepatitis C in Pakistan 2009. Pak J Gastroenterol. 2009;23(2):7–67.
10. Qureshi H. Prevalence of hepatitis B & C in Pakistan. Islamabad: Pakistan Medical Research Council; 2008.
11. Frank C, Mohamed MK, Strickland GT, Lavanchy D, Arthur RR, Magder LS, et al. The role of parenteral antischistosomal therapy in the spread of hepatitis C virus in Egypt. Lancet. 2000 Mar 11;355(9207):887–91.
12. Amon JJ, Garfein RS, Ahdieh-Grant L, Armstrong GL, Ouellet LJ, Latka MH, et al. Prevalence of hepatitis C virus infection among injection drug users in the United States, 1994–2004. Clin Infect Dis. 2008 Jun 15;46(12):1852–8.
13. Xia X, Luo J, Bai J, Yu R. Epidemiology of hepatitis C virus infection among injection drug users in China: systematic review and meta-analysis. Public Health. 2008 Oct;122(10):990–1003.
14. Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med. 2001 Jul 5;345(1):41–52.
15. Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005 Sep;5(9):558–67.
16. Seeff LB. Natural history of chronic hepatitis C. Hepatology. 2002 Nov;36(5 Suppl 1):S35-46.
17. Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol. 2014 Nov;61(1 Suppl):S45-57.
18. Kandeel A, Genedy M, El-Refai S, Funk AL, Fontanet A, Talaat M. The prevalence of hepatitis C virus infection in Egypt 2015: implications for future policy on prevention and treatment. Liver Int. 2017 Jan;37(1):45–53.
19. Tohme RA, Holmberg SD. Is sexual contact a major mode of hepatitis C virus transmission? Hepatology. 2010 Oct;52(4):1497–505.
20. Vandelli C, Renzo F, Romanò L, Tisminetzky S, De Palma M, Stroffolini T, et al. Lack of evidence of sexual transmission of hepatitis C among monogamous couples: results of a 10-year prospective follow-up study. Am J Gastroenterol. 2004 May;99(5):855–9.
21. Puoti C, Guarisco R, Spilabotti L, Bellis L, Mitidieri Costanza O, Dell’ Unto O, et al. Should we treat HCV carriers with normal ALT levels? The “5Ws” dilemma. J Viral Hepat. 2012 Apr;19(4):229–35.
22. Kane A, Lloyd J, Zaffran M, Simonsen L, Kane M. Transmission of hepatitis B, hepatitis C and human immunodeficiency viruses through unsafe injections in the developing world: model-based regional estimates. Bull World Health Organ. 1999;77(10):801–7.
23. Khan AJ, Luby SP, Fikree F, Karim A, Obaid S, Dellawala S, et al. Unsafe injections and the transmission of hepatitis B and C in a periurban community in Pakistan. Bull World Health Organ. 2000;78(8):956–63.
24. Kaldor JM, Dore GJ, Correll PK. Public health challenges in hepatitis C virus infection. J Gastroenterol Hepatol. 2000 May;15 Suppl:E83–90.
25. World Health Organization. Global surveillance and control of hepatitis C. Report of a WHO Consultation organized in collaboration with the Viral Hepatitis Prevention Board, Antwerp, Belgium. J Viral Hepat. 1999 Jan;6(1):35–47.
26. Janjua NZ, Nizamy M a. M. Knowledge and practices of barbers about hepatitis B and C transmission in Rawalpindi and Islamabad. JPMA J Pak Med Assoc. 2004 Mar;54(3):116–9.
27. World Health Organization. Global alert and response (GAR). Hepatitis C. [Internet]. Geneva: World Health Organization; 2002. Available from: http://www.who.int/csr/disease/hepatitis/whocdscsrlyo2003/en/index4.html.
28. Terrault NA, Dodge JL, Murphy EL, Tavis JE, Kiss A, Levin TR, et al. Sexual transmission of hepatitis C virus among monogamous heterosexual couples: the HCV partners study. Hepatology. 2013 Mar;57(3):881–9.
29. Centers for Disease Control and Prevention. Viral hepatitis surveillance — United States, 2009 [Internet]. [cited 2017 May 2]. Available from: https://www.cdc.gov/hepatitis/Statistics/2009Surveillance/Commentary.htm.
30. Chen SL, Morgan TR. The natural history of hepatitis C virus (HCV) infection. Int J Med Sci. 2006 Apr 1;3(2):47–52.
31. Freeman AJ, Dore GJ, Law MG, Thorpe M, Von Overbeck J, Lloyd AR, et al. Estimating progression to cirrhosis in chronic hepatitis C virus infection. Hepatology. 2001 Oct;34(4 Pt 1):809–16.
32. Levine RA, Sanderson SO, Ploutz-Snyder R, Murray F, Kay E, Hegarty J, et al. Assessment of fibrosis progression in untreated Irish women with chronic hepatitis C contracted from immunoglobulin anti-D. Clin Gastroenterol Hepatol. 2006 Oct;4(10):1271–7.
33. Jacobson IM, Davis GL, El-Serag H, Negro F, Trépo C. Prevalence and challenges of liver diseases in patients with chronic hepatitis C virus infection. Clin Gastroenterol Hepatol. 2010 Nov;8(11):924–933; quiz e117.
34. Beinhardt S, Aberle JH, Strasser M, Dulic-Lakovic E, Maieron A, Kreil A, et al. Serum level of IP-10 increases predictive value of IL28B polymorphisms for spontaneous clearance of acute HCV infection. Gastroenterology. 2012 Jan;142(1):78–85.e2.
35. Vogt M, Lang T, Frösner G, Klingler C, Sendl AF, Zeller A, et al. Prevalence and clinical outcome of hepatitis C infection in children who underwent cardiac surgery before the implementation of blood-donor screening. N Engl J Med. 1999 Sep 16;341(12):866–70.
36. Di Bisceglie AM. Hepatitis C and hepatocellular carcinoma. Hepatology. 1997 Sep;26(3 Suppl 1):34S–38S.
37. Kim WR. The burden of hepatitis C in the United States. Hepatology. 2002 Nov;36(5 Suppl 1):S30-34.
38. World Health Organization. Essential medicines and health products. First WHO prequalified hepatitis C rapid test opens the door to expanded treatment [press release] [Internet]. World Health Organization; 2016 [cited 2016 Dec 13]. Available from: http://www.who.int/medicines/news/prequal_hvc/en/.
39. Pockros PJ. Interferon-free hepatitis C therapy: how close are we? Drugs. 2012 Oct 1;72(14):1825–31.
40. Freeman JAD, Hill A. The use of generic medications for hepatitis C. Liver Int. 2016 Jul;36(7):929–32.
41. Hill A, Simmons B, Gotham D, Fortunak J. Rapid reductions in prices for generic sofosbuvir and daclatasvir to treat hepatitis C. J Virus Erad. 2017 Jan 25;2(1):28–31.
42. Andrieux-Meyer I, Cohn J, de Araújo ESA, Hamid SS. Disparity in market prices for hepatitis C virus direct-acting drugs. Lancet Glob Health. 2015 Nov;3(11):e676-677.
43. Freeman J, Sallie R, Kennedy A, Hieu PTN, Freeman J, Jeffreys G, et al. High sustained virological response rates using generic direct acting antiviral treatment for hepatitis C, imported into Australia. J Hepatol. 2016 Jan 1;64(2):S209.
44. World Health Organization. Combating hepatitis B and C to reach elimination by 2030: advocacy brief [Internet]. Geneva: World Health Organization; 2016. Available from: http://www.who.int/hepatitis/publications/hep-elimination-by-2030-brief/en/.
45. Nguyen-Khac E. [Results and place of Fibroscan in the non-invasive diagnosis of hepatic fibrosis]. Rev Med Interne. 2007 Feb;28(2):94–102.
46. European Association for Study of Liver, Asociacion Latinoamericana para el Estudio del Higado. EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015 Jul;63(1):237–64.
47. Houot M, Ngo Y, Munteanu M, Marque S, Poynard T. Systematic review with meta-analysis: direct comparisons of biomarkers for the diagnosis of fibrosis in chronic hepatitis C and B. Aliment Pharmacol Ther. 2016 Jan;43(1):16–29.
48. Spera AM, Eldin TK, Tosone G, Orlando R. Antiviral therapy for hepatitis C: Has anything changed for pregnant/lactating women? World J Hepatol. 2016 Apr 28;8(12):557–65.
49. University of Liverpool. Hepatitis drug interactions [Internet]. HEP Drug Interactions. 2017 [cited 2017 Jan 25]. Available from: http://www.hep-druginteractions.org/.
50. Lalezari JP, Nelson DR, Hyland RH, Lin M, Rossi SJ, Symonds WT, et al. Once daily sofosbuvir plus ribavirin for 12 and 24 weeks in treatment-naïve patients with HCV infection: the Quantum study [abstract, International Liver Congress 2013: 48th Annual Meeting of the European Association for the Study of the Liver, Amsterdam, Netherlands, 24 April–28 April 2013]. J Hepatol. 2013 Apr 1;58(Supplement 1):S346.
51. Osinusi A, Meissner EG, Lee Y-J, Bon D, Heytens L, Nelson A, et al. Sofosbuvir and ribavirin for hepatitis C genotype 1 in patients with unfavorable treatment characteristics: a randomized clinical trial. JAMA. 2013 Aug 28;310(8):804–11.
52. Gane EJ, Stedman CA, Hyland RH, Ding X, Svarovskaia E, Symonds WT, et al. Nucleotide polymerase inhibitor sofosbuvir plus ribavirin for hepatitis C. N Engl J Med. 2013 Jan 3;368(1):34–44.
53. Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013 May 16;368(20):1878–87.
54. Jacobson IM, Gordon SC, Kowdley KV, Yoshida EM, Rodriguez-Torres M, Sulkowski MS, et al. Sofosbuvir for hepatitis C Genotype 2 or 3 in patients without treatment options. N Engl J Med. 2013 May 16;368(20):1867–77.
55. FDA. U.S. Food & Drug Administration. FDA news release. FDA approves Epclusa for treatment of chronic hepatitis C virus infection [press release] [Internet]. Silver Spring : United States Food and Drug Administration; 2016 [cited 2017 Jan 26]. Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm508915.htm.
56. Feld JJ, Jacobson IM, Hézode C, Asselah T, Ruane PJ, Gruener N, et al. Sofosbuvir and velpatasvir for HCV genotype 1, 2, 4, 5, and 6 infection. N Engl J Med. 2015 Dec 31;373(27):2599–607.
57. Foster GR, Afdhal N, Roberts SK, Bräu N, Gane EJ, Pianko S, et al. Sofosbuvir and velpatasvir for HCV genotype 2 and 3 infection. N Engl J Med. 2015 Dec 31;373(27):2608–17.
58. Curry MP, O’Leary JG, Bzowej N, Muir AJ, Korenblat KM, Fenkel JM, et al. Sofosbuvir and velpatasvir for HCV in patients with decompensated cirrhosis. N Engl J Med. 2015 Dec 31;373(27):2618–28.
59. Poordad F, Lawitz E, Gutierrez JA, Evans B, Howe A, Feng H-P, et al. C-swift: grazoprevir/elbasvir + sofosbuvir in cirrhotic and noncirrhotic, treatment-naive patients with hepatitis C virus genotype 1 infection, for durations of 4, 6 or 8 weeks and genotype 3 infection for durations of 8 or 12 weeks — abstract O006. J Hepatol. 2015 Apr 1;62:S192–3.
60. Gerlach JT, Diepolder HM, Zachoval R, Gruener NH, Jung M-C, Ulsenheimer A, et al. Acute hepatitis C: high rate of both spontaneous and treatment-induced viral clearance. Gastroenterology. 2003 Jul;125(1):80–8.
61. Lehmann M, Meyer MF, Monazahian M, Tillmann HL, Manns MP, Wedemeyer H. High rate of spontaneous clearance of acute hepatitis C virus genotype 3 infection. J Med Virol. 2004 Jul;73(3):387–91.
62. Nomura H, Sou S, Tanimoto H, Nagahama T, Kimura Y, Hayashi J, et al. Short-term interferon-alfa therapy for acute hepatitis C: a randomized controlled trial. Hepatology. 2004 May;39(5):1213–9.
63. Jaeckel E, Cornberg M, Wedemeyer H, Santantonio T, Mayer J, Zankel M, et al. Treatment of acute hepatitis C with interferon alfa-2b. N Engl J Med. 2001 Nov 15;345(20):1452–7.
64. Santantonio T, Fasano M, Sinisi E, Guastadisegni A, Casalino C, Mazzola M, et al. Efficacy of a 24-week course of PEG-interferon alpha-2b monotherapy in patients with acute hepatitis C after failure of spontaneous clearance. J Hepatol. 2005 Mar;42(3):329–33.
65. Kamal S, Madwar M, He Q, Koziel M. Peginterferon alpha compared to conventional interferon alpha plus ribavirin combination therapy in symptomatic acute hepatitis C: a randomized trial of treatment onset, duration and cost effectiveness [abstract presented at 55th Annual Meeting of the American Association for the Study of Liver Diseases, Boston, Massachusetts, October 29–November 2, 2004]. Hepatology. 2004;40(Supplement S4):178A.
66. Deterding K, Grüner N, Buggisch P, Wiegand J, Galle PR, Spengler U, et al. Delayed versus immediate treatment for patients with acute hepatitis C: a randomised controlled non-inferiority trial. Lancet Infect Dis. 2013 Jun;13(6):497–506.
67. Mack CL, Gonzalez-Peralta RP, Gupta N, Leung D, Narkewicz MR, Roberts EA, et al. NASPGHAN practice guidelines: Diagnosis and management of hepatitis C infection in infants, children, and adolescents. J Pediatr Gastroenterol Nutr. 2012 Jun;54(6):838–55.
68. Wirth S, Lang T, Gehring S, Gerner P. Recombinant alfa-interferon plus ribavirin therapy in children and adolescents with chronic hepatitis C. Hepatology. 2002 Nov 1;36(5):1280–4.
69. Wirth S, Pieper-Boustani H, Lang T, Ballauff A, Kullmer U, Gerner P, et al. Peginterferon alfa-2b plus ribavirin treatment in children and adolescents with chronic hepatitis C. Hepatology. 2005 May;41(5):1013–8.
70. Wirth S, Ribes-Koninckx C, Calzado MA, Bortolotti F, Zancan L, Jara P, et al. High sustained virologic response rates in children with chronic hepatitis C receiving peginterferon alfa-2b plus ribavirin. J Hepatol. 2010 Apr;52(4):501–7.
71. Sokal EM, Bourgois A, Stéphenne X, Silveira T, Porta G, Gardovska D, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection in children and adolescents. J Hepatol. 2010 Jun;52(6):827–31.
72. González-Peralta RP, Kelly DA, Haber B, Molleston J, Murray KF, Jonas MM, et al. Interferon alfa-2b in combination with ribavirin for the treatment of chronic hepatitis C in children: efficacy, safety, and pharmacokinetics. Hepatology. 2005 Nov;42(5):1010–8.
73. El-Guindi MA. Hepatitis C Viral Infection in Children: Updated Review. Pediatr Gastroenterol Hepatol Nutr. 2016 Jun;19(2):83–95.
74. Hill A, Khoo S, Simmons B, Ford N. What is the minimum cost per person to cure HCV? In Kuala Lumpur, Malaysia: International Aids Society; 2013 [cited 2017 Jan 27]. Available from: http://pag.ias2013.org/abstracts.aspx?aid=3142.
75. World Health Organization. Notes on the design of a bioequivalance study: sofosbuvir. Guidance document, 13 October 2015 [Internet]. World Health Organization; 2015 [cited 2017 Jan 5]. Available from: https://extranet.who.int/prequal/.
76. Hill A, Golovin S, Dragunova J, Korologou-Linden RS. Virological response rates using generic direct acting antiviral treatment for hepatitis C, legally imported into Russia. AASLD Hepatol Poster Sess IV Abstr 1638-2112 [Internet]. 2017 Jan 25 [cited 2017 Jan 25]; Available from: http://onlinelibrary.wiley.com/doi/10.1002/hep.28800/full.
77. Derbala MF, Elsayad E, Hajelssedig O, Amer A, Eldweik N, Alkaabi SR, et al. Generic versus brand sofosbuvir-based therapy: safety and efficacy, real life data. AASLD Hepatol Poster Sess IV Abstr 1638-2112. 2016 Oct 1;64:811–1050.
78. Ogata N, Alter HJ, Miller RH, Purcell RH. Nucleotide sequence and mutation rate of the H strain of hepatitis C virus. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3392–6.
79. Bartenschlager R, Lohmann V. Replication of hepatitis C virus. J Gen Virol. 2000 Jul;81(Pt 7):1631–48.
80. Ray SC, Thomas DL. Hepatitis C. In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas, and Bennett’s principles and practice of infectious diseases. 8th ed. Philadelphia: Elsevier Saunders; 2014. p. 1904–27.
81. Pawlotsky J-M. Hepatitis C virus resistance to direct-acting antiviral drugs in interferon-free regimens. Gastroenterology. 2016 Jul;151(1):70–86.
82. Ahmed A, Felmlee DJ. Mechanisms of hepatitis C viral resistance to direct acting antivirals. Viruses. 2015 Dec 18;7(12):6716–29.
83. Kattakuzhy S, Gross C, Teferi G, Jenkins V, Silk R, Akoth E, et al. A novel task shifting model to expand the HCV care continuum: the Ascend investigation [abstract of paper presented at International Liver Congress, Barcelona, Spain, 13–17 April 2015]. J Hepatol. 2016 Jan 1;64(2):S224–5.
84. Arora S, Thornton K, Murata G, Deming P, Kalishman S, Dion D, et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med. 2011 Jun 9;364(23):2199–207.



























