Strongyloidiasis is an infection with Strongyloides stercoralis (Fig. 1), a roundworm that occurs widely in tropical and subtropical areas, but also in countries with temperate climates (Table 3).
- Human strongyloidiasis is caused by two species of the parasitic nematode Strongyloides. Of these, S. stercoralis is the most common pathogen for humans; S. fuelleborni is found sporadically in Africa and Papua New Guinea.
- Infective S. stercoralis larvae can replicate in the bowel and directly autoinfect positive individuals—autoinfection is the major issue that differentiates strongyloidiasis from other soil-transmitted helminthiasis (STH) macroparasite infection. The main species that infect people are the roundworm (Ascaris lumbricoides), the whipworm (Trichuris trichiura), and hookworms (Necator americanus and Ancylostoma duodenale) [1].
- The adult male worm is found only in the soil. It is not a tissue parasite and is not found in the human host.
- The adult female worm is very small and almost transparent. It measures approximately 2.2–2.5 mm in length, with a diameter of 50 µm, and it lives in tunnels between the enterocytes in the human small bowel.
- Infective larvae can replicate in the contaminated soil and infect exposed individuals.
Strongyloidiasis is different from all other soil-transmitted helminthic infections because the eggs produced through parthenogenesis by the parasitic female worm hatch when still in the bowel and produce rhabditiform larvae.
- The larvae are usually excreted in the feces, but some can mature to the filariform stage and reinfect the host by penetrating the last part of the bowel or the perianal skin (autoinfective cycle).
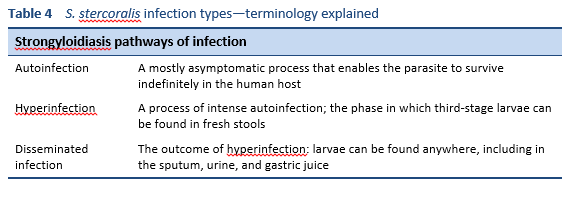
- Depending on the host immune response, this can lead to dissemination and hyperinfection (Table 4).

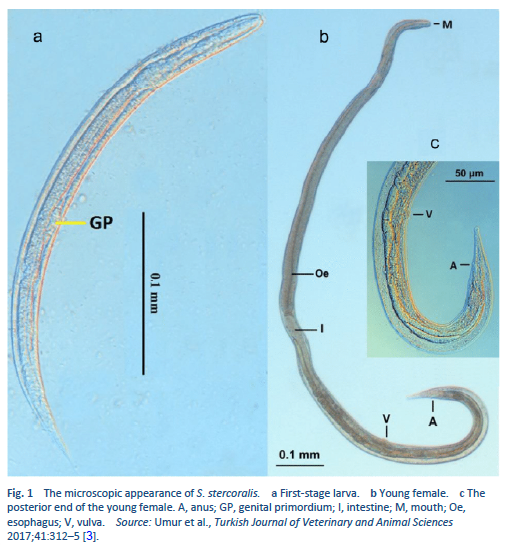
There are two important stages in the life cycle of the worm, the rhabditiform stage and the filariform stage (Figs. 2, 3).
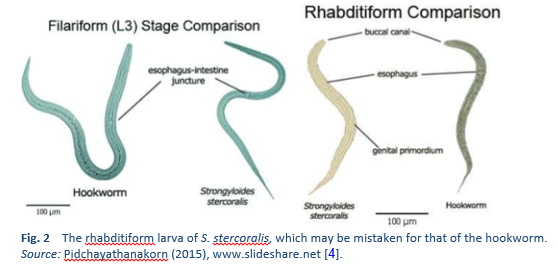
2.1 Soil-transmitted helminthiases and strongyloidiasis
Although strongyloidiasis has a similar route of infection to the other soil-transmitted helminthiases, it needs additional diagnostic tools beyond microscopy and requires different treatment. In areas in which preventive anthelmintic chemotherapy with ivermectin has been used to control onchocerciasis or lymphatic filariasis, there has been a noticeable reduction in the prevalence of strongyloidiasis [6–10]. The WHO Essential Medicines Committee has included ivermectin in its list, including in combination with albendazole, for strongyloidiasis. Some 900 million people are now receiving this combination as part of neglected tropical disease (NTD) campaigns [11].
2.2 Pathophysiology
Strongyloides stercoralis has a unique and complex life cycle. Figure 4 outlines the unique routes of S. stercoralis replication.
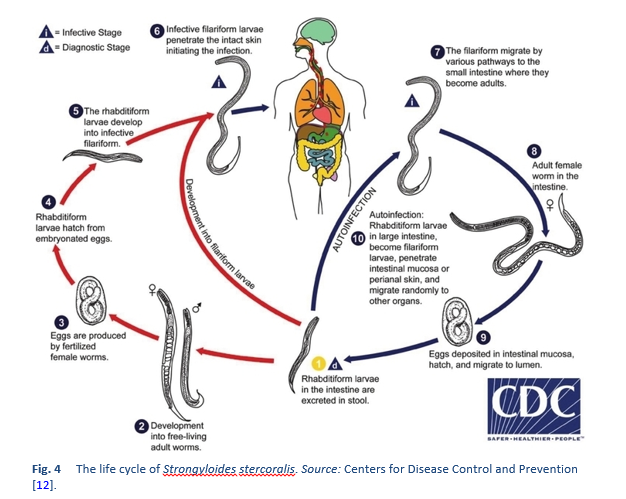
The life cycle of Strongyloides is more complex than that of most nematodes, with its alternation between free-living and parasitic cycles and its potential for autoinfection and multiplication within the host. There are two types of cycle:
- The free-living cycle. The rhabditiform larvae passed in the stool can either molt twice and become infective filariform larvae (direct development), or molt four times and become free-living adult males and females that mate and produce eggs from which rhabditiform larvae hatch. The latter in turn can either develop into a new generation of free-living adults or into infective filariform larvae. The filariform larvae penetrate the human host’s skin to initiate the parasitic cycle.
The free-living stage of the nematode’s life cycle is limited to a maximum of one generation [13]. This is a unique feature of strongyloidiasis that has important implications both for treating infected people and for environmental control in preventing transmission. This means that it is vital that eradication therapy must be highly effective in order to remove all viable forms of the organism from the infected individual.
- The parasitic cycle. Filariform larvae in contaminated soil penetrate the human skin and are transported to the lungs, where they penetrate the alveolar spaces; they are carried through the bronchial tree to the pharynx, are swallowed, and then reach the small intestine. In the small intestine, they molt twice and become adult female worms. The females live threaded in the epithelium of the small intestine and through parthenogenesis produce eggs, which yield rhabditiform larvae. The rhabditiform larvae can either be passed in the stool (see “free-living cycle” above) or can develop further and cause autoinfection. In autoinfection, the rhabditiform larvae become infective filariform larvae, which can penetrate either the intestinal mucosa (internal autoinfection) or the skin of the perianal area (external autoinfection); in either case, the filariform larvae may follow the previously described route, being carried successively to the lungs, the bronchial tree, the pharynx, and the small intestine, where they mature into adults; or they may disseminate widely in the body. To date, occurrence of autoinfection in humans with helminthic infections is recognized only in Strongyloides stercoralis and Capillaria philippinensis infections. S. stercoralis is much more common and widespread. In strongyloidiasis, autoinfection explains the existence of infections persisting in persons who have not been in an endemic area for many years (the current record is 65 years) and the life-threatening morbidity of hyperinfection in immunocompromised individuals—both unusual for worm infections.
2.3 Disease burden and endemicity
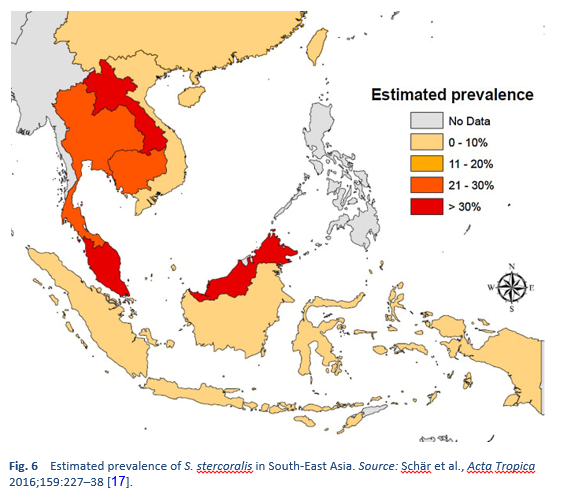
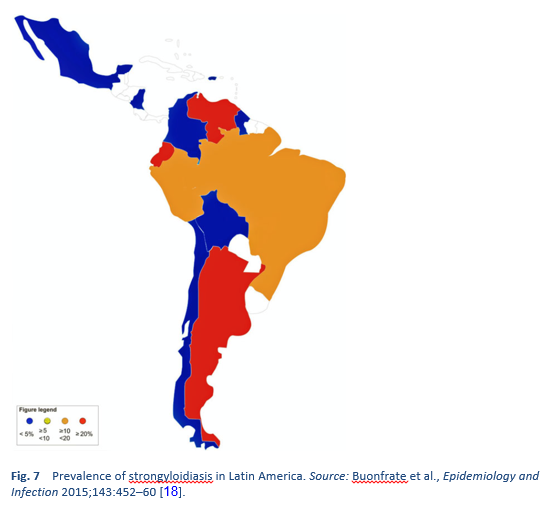
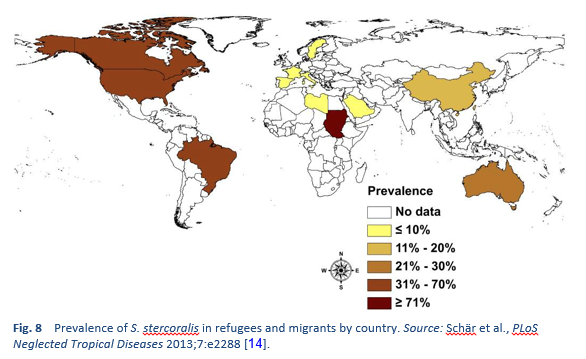
Strongyloidiasis is endemic in tropical and subtropical regions (Figs. 5–8), and the prevalence is probably much higher than the 100 million people previously quoted: higher estimates of up to 370 million people have been published [2]. It is also widespread in eastern Europe, and scattered foci of the infection have been reported in elderly people in the Mediterranean region.
We know little about the prevalence of infection and less about the clinical burden of morbidity. If it is indeed widespread, the risk of iatrogenic hyperinfection (with immunosuppressive management) is a challenge. It is thought that strongyloidiasis infects up to 40% of the population in some areas of the tropics and subtropics [14].
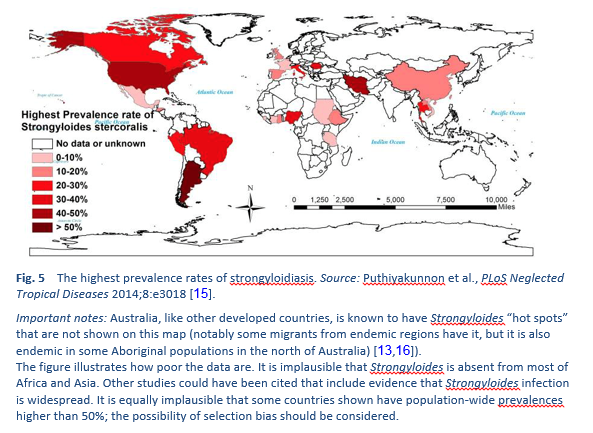
Migrant infections may occur in any country and may represent a potential global hazard. The disease can present in a consulting room anywhere.
2.4 Risk factors and special groups at risk of infection
The biggest risk factor overall is socio-economic disadvantage in a relevant environment in which Strongyloides is endemic.
- Poverty, poor housing, poor sanitation; walking barefoot, living in an environment in which open defecation occurs
- Prisoners of war
- Refugee status—refugees from countries in which strongyloidiasis is endemic
- Travelers to and from endemic areas
- Some studies have reported male sex, advanced age, animal–human transmission, and humid, wet climates in the tropics and subtropics as risk factors [17]
2.5 Risk factors and special groups at risk for disseminated infection
- Immunosuppressive medication—especially corticosteroids, but also tacrolimus and chemotherapeutic agents
- Patients with altered cellular immunity
- Human T-lymphotropic virus type 1 infection
- Neoplasms, particularly hematologic malignancies (lymphoma, leukemia)
- Organ transplantation (kidney allograft recipients)
- Minor/possible risk factors: collagen vascular disease, malabsorption and malnutrition states, end-stage renal disease, diabetes mellitus, local host factors, diverticular and blind loops (persistent strongyloidiasis in a blind loop in the intestine)
2.6 Strongyloidiasis and immunosuppressed patients
Strongyloidiasis ranges from asymptomatic to severe forms and can lead to hyperinfection syndrome and disseminated disease, associated with a high mortality rate in immunosuppressed patients.
In the tropics, there are many patients with rheumatoid arthritis, bronchial asthma, and glomerulonephritis who receive long-term steroid treatment. Patients can purchase steroids directly from pharmacies.
Strongyloidiasis is not an important AIDS-associated opportunistic infection, but it is an opportunistic infection associated with human T-lymphotropic virus type I (HTLV-I) [19]. Although patients with human immunodeficiency virus/acquired immune deficiency syndrome (HIV/AIDS) can have disseminated strongyloidiasis or hyperinfection syndrome, observational studies have not shown an increased risk in this population [20].
2.7 Mortality and morbidity
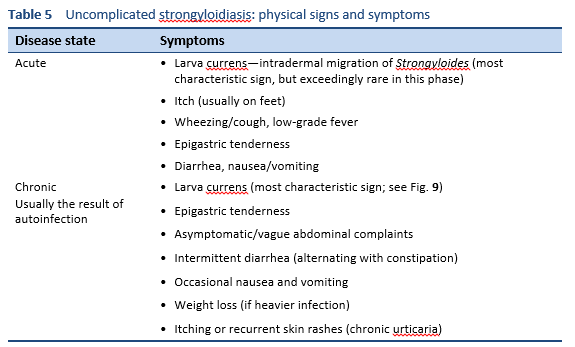
Acute strongyloidiasis is often asymptomatic and can remain hidden for decades. Immunocompetent patients often have asymptomatic chronic lifelong infections if untreated.
Chronic infections are a potentially important cause of undisclosed morbidity. There is also a lack of efficient diagnostic tools, which are often cumbersome and have low sensitivity, so that the true prevalence of infection and morbidity is not known. Since strongyloidiasis is viewed as an unusual disease, there has been little investment in diagnostic or epidemiological surveys, especially in children.
Clinically apparent strongyloidiasis can lead to cutaneous, gastrointestinal, and pulmonary symptoms.
Evidence highlights the need to survey patients with eosinophilia even when a history of residence or travel in an endemic area is absent [22].
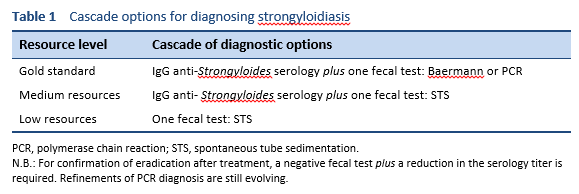
Several diagnostic procedures have been developed over the years, and their use depends on local availability and relevant expertise: string tests, duodenal aspirates, duodenal biopsy, bronchoalveolar lavage (BAL), immunodiagnostic tests, and repeated examination of fresh stool with different methods.
The global prevalence of Strongyloides stercoralis infection has long been underestimated. This is likely due to reliance on direct stool microscopy and the Kato–Katz technique, which are commonly used in prevalence studies but are inadequate for S. stercoralis detection [23]. The commonly used fecal-based methods have particularly low sensitivity. Microscopy can be improved by examination of several stool samples, as well as concentration techniques [24], but the sensitivity remains low.
In both low/middle-income and also developed countries, the number of personnel who are well trained in the microscopic identification of parasites appears to be decreasing.
- The application of molecular assays, still lagging behind virology or bacteriology, is expected to increase in parasitology.
- Molecular diagnosis of S. stercoralis infection has yet to demonstrate optimal sensitivity.
- Molecular diagnosis is unlikely to completely replace the other diagnostic techniques.
- Serological assays currently show the highest sensitivity and are important for screening of S. stercoralis and assessment of cure [23].
Lodh et al. [25] presented research results showing that S. stercoralis DNA can be detected in urine. Once available, and if they are sufficiently sensitive, urine sample tests may be attractive, as they are much less labor-intensive and resource-intensive and do not involve the health risk of examining fresh stool [25].
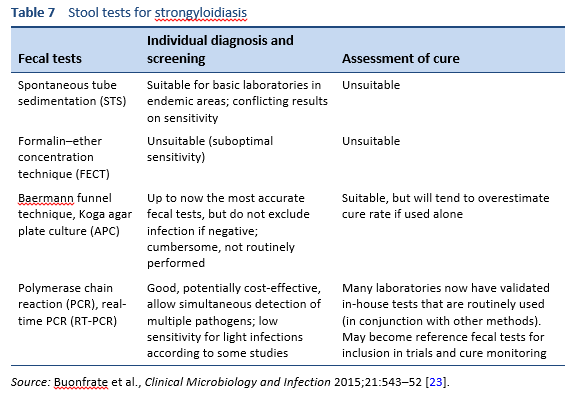
4.1 Stool tests
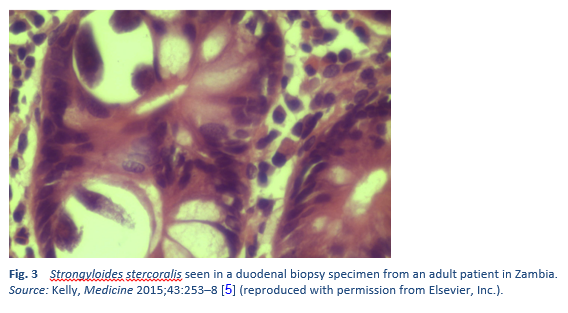
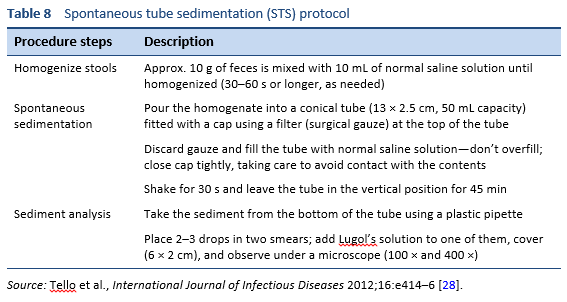
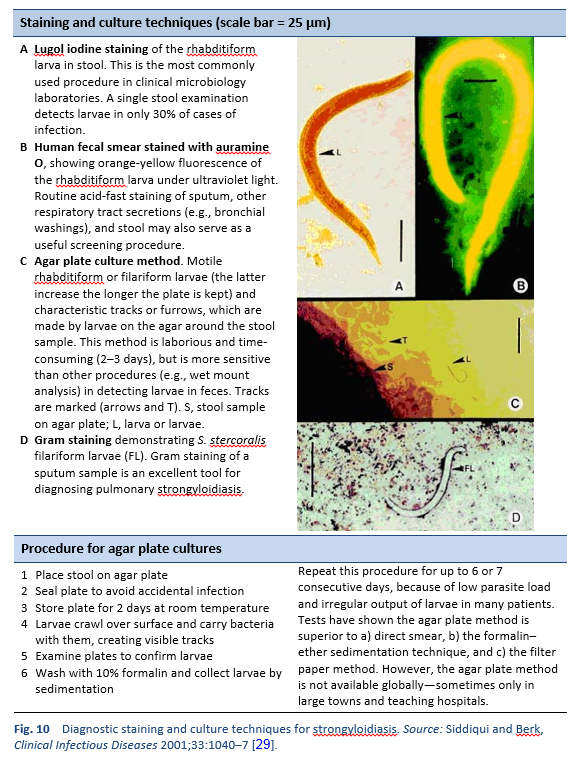
Finding the larvae in stool, duodenal fluid, or occasionally in other tissues or fluids by means of microscopy establishes a definitive diagnosis of strongyloidiasis (Tables 7, 8; Figs. 10, 11). However, because of low larval densities, a single examination is insensitive [26].
Several methods are used to identify larvae in stool by microscopy:
- Microscopy after concentration
- Baermann funnel technique (still regarded as the gold standard)
- Formalin–ether concentration technique (FECT)
- Harada–Mori filter paper culture
- Koga agar plate culture
- Use of a dissecting microscope to visualize larvae on agar plates
- Direct smear of feces in saline–Lugol iodine stain
The use of these methods depends on local resource availability and especially the expertise of the microscopist.
Stool analyses for Strongyloides using the Baermann funnel technique and Koga agar culture method are the best fecal diagnostic methods for field settings today. These methods detect the parasite with greater sensitivity than other fecal methods.
- PCR is promising, but not yet standardized; there are concerns about the sensitivity of PCR, as it varies across different studies.
- Anamnart et al. [27] tested stimulation of excretion of S. stercoralis larvae in stool by oral administration of a single dose of 400 mg albendazole and suggested that the application of albendazole plus the modified formalin–ether concentration technique (MFECT) could be used in patients with suspected asymptomatic strongyloidiasis—including patients with unexplained chronic diarrhea, patients returning from areas where strongyloidiasis is endemic, and patients with negative results in other parasitological tests [27].

4.2 Serodiagnosis of strongyloidiasis
In comparison with the Baermann technique and agar plate culture, serological tests have greater sensitivity, although some authors have concerns about their specificity [20].
- Many serological tests cross-react with filarial parasites, schistosomes, and Ascaris lumbricoides, decreasing the specificity of the tests.
- It can be difficult to distinguish between active cases and historical cases, as antibodies can persist for some time.
- More specific serological tests using recombinant antigens have been and are continuing to be developed and are available at specific laboratories.
- Serologic tests typically show a significant drop in titer by 6–12 months after parasite eradication, so that they can be used to assess cure [20].
The most convenient and widely used serological method is the enzyme-linked immunosorbent assay (ELISA) to detect serum immunoglobulin G (IgG) against a crude extract of filariform larvae. ELISA is labor-intensive and requires a certain level of laboratory infrastructure for performance and interpretation of results, and this has hampered its applicability especially in areas where Strongyloides is endemic [26]. Moreover, serology has limited value for follow-up after cure in endemic areas, as reinfection is possible.
4.3 Differential diagnosis
There are many conditions that produce similar symptoms, including causes of acute and chronic diarrhea and malabsorption, other causes of eosinophilia, and other causes of severe Gram-negative septicemia. The following should be considered in the differential diagnosis:
- Intestinal infections—amebiasis, bacterial colitis, Shigella, Campylobacter, Yersinia, Clostridium difficile; see the WGO Global Guideline on Acute Diarrhea, Table 4 [30].
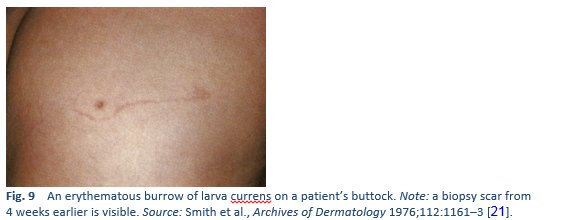
- Non-human hookworm infection, producing cutaneous larva migrans—distinguished from the larva currens caused by S. stercoralis by the absence of scabbing, rapid migration, perianal involvement and wide band of urticaria in larva currens.
- Inflammatory bowel disease.
- Irritable bowel syndrome.
- Functional abdominal disorders.
- Drugs—nonsteroidal anti-inflammatory drugs (NSAIDs) and many others—are possible causes of eosinophilia.
The key diagnostic element is to think of strongyloidiasis as a possible diagnosis and identify the parasite directly and/or through serologic/molecular tests.
- Spontaneous cure cannot be expected, due to the parasite’s unique autoinfection life cycle.
- Treat all patients with strongyloidiasis, even when asymptomatic, because of the risk of hyperinfection—a potentially fatal complication.
- Reliable diagnosis of patients at risk is needed for accurate recognition and treatment before immunosuppressive therapy is initiated, or in patients with HTLV-I or human immunodeficiency virus (HIV) infection.
- If emergency immunosuppression is required in a patient who may have previously undiagnosed strongyloidiasis, and diagnostic tests are not rapidly available (very few hospitals can do same-day serology), presumptive treatment with ivermectin should be considered.
- Cure can be achieved with single-dose ivermectin.
- Failure of treatment with ivermectin is generally due to the impairment of host immunity (frequent in patients with HTLV-I infection) [26,31].
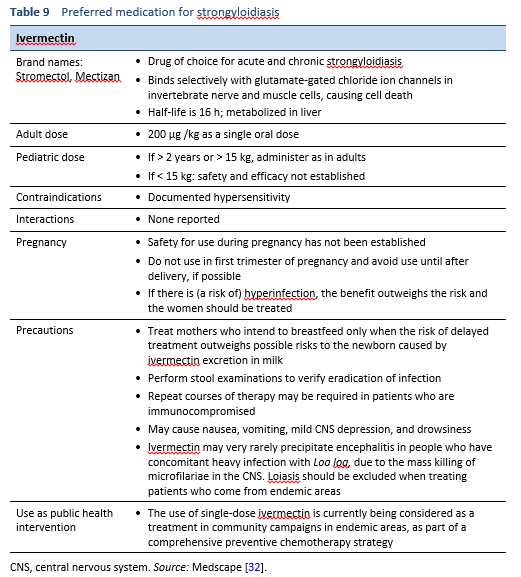
5.1 Uncomplicated strongyloidiasis
The treatment of strongyloidiasis (Table 9) is difficult because in contrast to other helminth infections, the Strongyloides worm burden has to be eradicated completely.
- Complete eradication is difficult to ascertain, because of the low worm load and irregular larval output.
- A definitive cure cannot be established on the basis of a negative follow-up stool examination alone—it also requires a decline in both serological titers and eosinophilia.
- A single stool analysis for strongyloidiasis was found to be negative in up to 70% of known cases of Strongyloides infection. Reliable testing requires multiple stool examinations, probably at least three and with suitable techniques.
- In the tropics, follow-up is a problem and if only fecal testing is available, it becomes the method of choice.
- Albendazole (400 mg b.i.d. for 3 days) is sometimes used as an alternative or compromise [33,34]. However, the efficacy of albendazole in the treatment of strongyloidiasis has been shown to be very low in comparison with ivermectin, and it should therefore not be used unless there is no alternative [35].
5.2 Hyperinfection or disseminated infection
Although some authors state that these terms describe two different aspects of the infection (hyperinfection: high levels of larvae in the usual body parts; dissemination: larvae present in any body part, not usually included in the parasitic cycle), they can probably be used interchangeably. In fact, they both refer to a very high parasite load and rapid spread of the infection—usually in immunosuppressed patients and often associated with corticosteroid treatment. Hyperinfection carries a high risk of Gram-negative septicemia, so broad-spectrum antibiotics are usually given, especially to prevent bacterial meningitis.
In critically ill people with hyperinfection or disseminated strongyloidiasis who are unable to take oral medicines, ivermectin has been administered successfully by the subcutaneous route [36]. For critically ill people, ivermectin is given daily for a duration of at least 14 days, with the total duration of treatment depending on when microscopic examination of body fluids positive for larvae become negative (this can be stool or urine, or others in cases of hyperinfection) [37].
5.3 Prevention and disease control
Infection is prevented by avoiding direct skin contact with soil containing infective larvae. People at risk, especially children, should wear footwear when walking on areas with infected soil. Patients at risk should be identified and appropriate diagnostic tests should be performed before they begin immunosuppressive therapy.
Persons in household contact with patients are not at risk for infection. The proper disposal of human excreta reduces the prevalence of strongyloidiasis substantially.
No accepted prophylactic regimen exists and no vaccine is available.
Standard precautions should be observed for patients hospitalized with strongyloidiasis. Wearing gloves and gowns and diligent handwashing hygiene is important for those coming into potential contact with the patient’s feces [20].
- Early detection and effective treatment of S. stercoralis infection.
- Screening of patients who are at risk for chronic strongyloidiasis before immunosuppressive treatment is started, especially with corticosteroids.
- Preventive chemotherapy (PC) for S. stercoralis infection is not yet recommended by WHO, nor is it included in the strategy for soil-transmitted helminth control. However, consistent side benefits on S. stercoralis prevalence have been demonstrated after lymphatic filariasis and onchocerciasis elimination programs that used repeated PC with ivermectin/albendazole or with ivermectin alone [38].
- Proper evaluation of treatment using stool examination (with highly sensitive tests such as the Baermann technique, filter-paper culture, and agar-plate culture) and specific IgG serology follow-up for 1–2 years [39].
- Overseas presumptive treatment programs in refugee populations from countries where intestinal parasites are endemic (hookworm, Trichuris trichiura, Ascaris lumbricoides, and Strongyloides stercoralis) [40].
- The installation and use of safe waste disposal systems still remains important [41].
- Wearing footwear could interrupt transmission of strongyloidiasis, but the cultural acceptability of footwear is low, particularly in hot climates, so other environmental control methods should be assessed [42]. People who don’t have shoes often don’t have chairs, and then the buttocks are an additional target.
- Detect anthelmintic resistance at an early stage. Various in vivo and in vitro methods are available for assessing the efficacy of anthelmintics, and specific laboratory methods can be applied to confirm a suspicion of resistance in the field—e.g., as described in the World Association for the Advancement of Veterinary Parasitology (WAAVP) study recommendations and guidelines [43–45].
The study by Forrer et al. [46] showed that community-based single-dose ivermectin treatment for S. stercoralis plus sanitation effectively reduced the infection risk in rural communities in Cambodia, with over 85% of villagers remaining negative 1 year after treatment. Infection control is feasible and highly beneficial, particularly in combination with improved sanitation [46].
Khieu et al. [47] found that individuals with a latrine at home were infected with S. stercoralis significantly less frequently than those without one. The calculated population attributable risk would be reduced by 39% if all participants used a latrine for defecation [17,47].
Croker and She noted that the high prevalence of eosinophilia among persons with latent Strongyloides infection in Los Angeles County highlights the importance of screening individuals with eosinophilia in whom more common causes have been ruled out [48].
The StrongNet [38], an international network for improving diagnosis and access to treatment for strongyloidiasis control, advocates better and field-friendly diagnosis as well as the availability of ivermectin on a large scale for the control of strongyloidiasis in endemic areas. Following the efforts of this network, ivermectin has recently been included to the WHO’s Essential Medicines List for the treatment of strongyloidiasis; the ultimate goal is to develop a public health control strategy and to include S. stercoralis in the WHO’s preventive chemotherapy strategy for soil-transmitted helminthiasis .
5.4 Prognosis
Acute and chronic strongyloidiasis have a good prognosis. However, untreated infection can persist for the remainder of the patient’s life, due to the autoinfection cycle. A patient’s prolonged absence from an endemic area is no guarantee of freedom from infection. Severe disseminated infection is commonly a fatal event, and it is often unresponsive to therapy.
In chronic strongyloidiasis, immunosuppression poses a risk for accelerated autoinfection. This may result in a sepsis-like syndrome, S. stercoralis hyperinfection, and the dissemination of larvae to distant organs such as the central nervous system, causing S. stercoralis–associated meningitis [49].
6.1 Abbreviations

6.2 Gold standard guideline
6.3 References
1. World Health Organization. Soil-transmitted helminth infections [Internet]. Geneva: World Health Organization; 2017 [accessed 2018 Mar 13]. Available from: http://www.who.int/mediacentre/factsheets/fs366/en/.
2. Bisoffi Z, Buonfrate D, Montresor A, Requena-Méndez A, Muñoz J, Krolewiecki AJ, et al. Strongyloides stercoralis: a plea for action. PLoS Negl Trop Dis 2013 May 9;7(5):e2214.
3. Umur Ş, Meral Y, Bölükbaş CS, Gürler AT, Açici M. First clinical Strongyloides stercoralis case in a dog in Turkey. Turk J Vet Anim Sci 2017;41:312–5.
4. Pidchayathanakorn P. Nemathelminthes [46 slides] [Internet] 2015 [accessed 2018 Mar 13]. Available from: https://www.slideshare.net/PaemikaPidchayathana/nemathelminthes-review.
5. Kelly P. Infectious diarrhoea. Med Abingdon 2015 May;43(5):253–8.
6. Knopp S, Mohammed KA, Rollinson D, Stothard JR, Khamis IS, Utzinger J, et al. Changing patterns of soil-transmitted helminthiases in Zanzibar in the context of national helminth control programs. Am J Trop Med Hyg 2009 Dec;81(6):1071–8.
7. Anselmi M, Buonfrate D, Guevara Espinoza A, Prandi R, Marquez M, Gobbo M, et al. Mass administration of ivermectin for the elimination of onchocerciasis significantly reduced and maintained low the prevalence of Strongyloides stercoralis in Esmeraldas, Ecuador. PLoS Negl Trop Dis 2015 Nov;9(11):e0004150.
8. Barda B, Albonico M, Buonfrate D, Ame SM, Ali S, Speich B, et al. Side benefits of mass drug administration for lymphatic filariasis on Strongyloides stercoralis. Prevalence on Pemba Island, Tanzania. Am J Trop Med Hyg 2017 Sep;97(3):681–3.
9. Bisoffi Z. Human strongyloidiasis: time to act? Paper presented at the 27th European Congress of Clinical Microbiology And Infectious Diseases (ECCMID), Vienna, April 2017. Basel, Switzerland: European Society of Clinical Microbiology and Infectious Diseases; 2017. (ESCMID eLibrary). Available from: www.escmid.org/escmid_publications/escmid_elibrary/material/?mid=44339.
10. World Health Organization. What are intestinal worms (soil transmitted helminthiasis)? [Internet]. Geneva: World Health Organization; [accessed 2018 Mar 13]. Available from: http://www.who.int/intestinal_worms/disease/en/.
11. Bundy DAP, Appleby LJ, Bradley M, Croke K, Hollingsworth TD, Pullan R, et al. Mass deworming programs in middle childhood and adolescence. In: Bundy DAP, de Silva N, Horton S, Jamison DT, Patton GC, editors. Child and Adolescent Health and Development [Internet]. 3rd ed. Washington, DC: International Bank for Reconstruction and Development / World Bank Group; 2017. p. 165–82. (Disease Control Priorities; vol. 8). Available from: http://dcp-3.org/chapter/2437/deworming.
12. Centers for Disease Control and Prevention. Parasites — Strongyloides [Internet]. Atlanta, GA: Centers for Disease Control and Prevention; 2015 [accessed 2018 Mar 13]. Available from: https://www.cdc.gov/parasites/strongyloides/biology.html.
13. Ross KE, Bradbury RS, Garrard TA, O’Donahoo FJ, Shield JM, Page W, et al. The National Strongyloides Working Group in Australia 10 workshops on: commendations and recommendations. Aust N Z J Public Health 2017 Jun;41(3):221–3.
14. Schär F, Trostdorf U, Giardina F, Khieu V, Muth S, Marti H, et al. Strongyloides stercoralis: global distribution and risk factors. PLoS Negl Trop Dis 2013;7(7):e2288.
15. Puthiyakunnon S, Boddu S, Li Y, Zhou X, Wang C, Li J, et al. Strongyloidiasis — an insight into its global prevalence and management. PLoS Negl Trop Dis 2014 Aug;8(8):e3018.
16. Kearns TM, Currie BJ, Cheng AC, McCarthy J, Carapetis JR, Holt DC, et al. Strongyloides seroprevalence before and after an ivermectin mass drug administration in a remote Australian Aboriginal community. PLoS Negl Trop Dis 2017 May;11(5):e0005607.
17. Schär F, Giardina F, Khieu V, Muth S, Vounatsou P, Marti H, et al. Occurrence of and risk factors for Strongyloides stercoralis infection in South-East Asia. Acta Trop 2016 Jul;159:227–38.
18. Buonfrate D, Mena MA, Angheben A, Requena-Mendez A, Muñoz J, Gobbi F, et al. Prevalence of strongyloidiasis in Latin America: a systematic review of the literature. Epidemiol Infect 2015 Feb;143(3):452–60.
19. Crompton DWT, Engels D, Savioli L, Montresor A, Neira M, editors. Preparing to control schistosomiasis and soil-transmitted helminthiasis in the twenty-first century [special double issue of journal]. Acta Trop 2003;86(2–3):121–347.
20. Centers for Disease Control and Prevention. Parasites — Strongyloides. Resources for health professionals [Internet]. Atlanta, GA: Centers for Disease Control and Prevention; 2016 [accessed 2018 Mar 13]. Available from: https://www.cdc.gov/parasites/strongyloides/health_professionals/index.html.
21. Smith JD, Goette DK, Odom RB. Larva currens. Cutaneous strongyloidiasis. Arch Dermatol 1976 Aug;112(8):1161–3.
22. Repetto SA, Ruybal P, Solana ME, López C, Berini CA, Alba Soto CD, et al. Comparison between PCR and larvae visualization methods for diagnosis of Strongyloides stercoralis out of endemic area: A proposed algorithm. Acta Trop 2016 May;157:169–77.
23. Buonfrate D, Formenti F, Perandin F, Bisoffi Z. Novel approaches to the diagnosis of Strongyloides stercoralis infection. Clin Microbiol Infect 2015 Jun;21(6):543–52.
24. Knopp S, Mgeni AF, Khamis IS, Steinmann P, Stothard JR, Rollinson D, et al. Diagnosis of soil-transmitted helminths in the era of preventive chemotherapy: effect of multiple stool sampling and use of different diagnostic techniques. PLoS Negl Trop Dis 2008;2(11):e331.
25. Lodh N, Caro R, Sofer S, Scott A, Krolewiecki A, Shiff C. Diagnosis of Strongyloides stercoralis: detection of parasite-derived DNA in urine. Acta Trop 2016 Nov;163:9–13.
26. van Doorn HR, Koelewijn R, Hofwegen H, Gilis H, Wetsteyn JCFM, Wismans PJ, et al. Use of enzyme-linked immunosorbent assay and dipstick assay for detection of Strongyloides stercoralis infection in humans. J Clin Microbiol 2007 Feb;45(2):438–42.
27. Anamnart W, Pattanawongsa A, Intapan PM, Maleewong W. Albendazole stimulates the excretion of Strongyloides stercoralis larvae in stool specimens and enhances sensitivity for diagnosis of strongyloidiasis. J Clin Microbiol 2010 Nov;48(11):4216–20.
28. Tello R, Terashima A, Marcos LA, Machicado J, Canales M, Gotuzzo E. Highly effective and inexpensive parasitological technique for diagnosis of intestinal parasites in developing countries: spontaneous sedimentation technique in tube. Int J Infect Dis 2012 Jun;16(6):e414-416.
29. Siddiqui AA, Berk SL. Diagnosis of Strongyloides stercoralis infection. Clin Infect Dis 2001 Oct 1;33(7):1040–7.
30. World Gastroenterology Organisation. Acute diarrhea in adults and children: a global perspective [Internet]. Milwaukee, WI: World Gastroenterology Organisation; 2012 [accessed 2018 Mar 13]. Available from: http://www.worldgastroenterology.org/guidelines/global-guidelines/acute-diarrhea/acute-diarrhea-english.
31. Varatharajalu R, Kakuturu R. Strongyloides stercoralis: current perspectives. Rep Parasitol 2016;(5):23–33.
32. Medscape. Ivermectin (Rx). Brand and other names: Stromectol. Dosage forms & strengths. [Internet]. Medscape; [accessed 2018 Mar 13]. Available from: https://reference.medscape.com/drug/stromectol-ivermectin-342657.
33. Horton J. Albendazole: a review of anthelmintic efficacy and safety in humans. Parasitology 2000;121 Suppl:S113-132.
34. Venkatesan P. Albendazole. J Antimicrob Chemother 1998 Feb;41(2):145–7.
35. Henriquez-Camacho C, Gotuzzo E, Echevarria J, White AC, Terashima A, Samalvides F, et al. Ivermectin versus albendazole or thiabendazole for Strongyloides stercoralis infection. Cochrane Database Syst Rev 2016 Jan 18;(1):CD007745.
36. Chiodini PL, Reid AJ, Wiselka MJ, Firmin R, Foweraker J. Parenteral ivermectin in Strongyloides hyperinfection. Lancet 2000 Jan 1;355(9197):43–4.
37. Boulware DR. Strongyloides infection. BMJ Best Pract [Internet]. Available from: http://bestpractice.bmj.com/best-practice/monograph/907/treatment/step-by-step.html.
38. Albonico M, Becker SL, Odermatt P, Angheben A, Anselmi M, Amor A, et al. StrongNet: An international network to improve diagnostics and access to treatment for strongyloidiasis control. PLoS Negl Trop Dis 2016 Sep;10(9):e0004898.
39. Luvira V, Watthanakulpanich D, Pittisuttithum P. Management of Strongyloides stercoralis: a puzzling parasite. Int Health 2014 Dec;6(4):273–81.
40. Maskery B, Coleman MS, Weinberg M, Zhou W, Rotz L, Klosovsky A, et al. Economic analysis of the impact of overseas and domestic treatment and screening options for intestinal helminth infection among US-bound refugees from Asia. PLoS Negl Trop Dis 2016 Aug;10(8):e0004910.
41. Nelson GS. [Review of D.I. Grove, A history of human helminthology (1990).]. J Helminthol 1991;65(2):120.
42. Ross KE, O’Donahoo FJ, Garrard TA, Taylor MJ. Simple solutions to Strongyloides stercoralis infection. BMJ Clin Res Ed 2013 Oct 22;347:f6294.
43. European Medicines Agency Committee for Medicinal Products for Veterinary Use (CVMP). Reflection paper on anthelmintic resistance [Internet]. London: European Medicines Agency; 2017 [accessed 2018 Mar 13]. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/general/general_content_001563.jsp&mid=WC0b01ac058002ddc2.
44. Wood IB, Amaral NK, Bairden K, Duncan JL, Kassai T, Malone JB, et al. World Association for the Advancement of Veterinary Parasitology (W.A.A.V.P.) second edition of guidelines for evaluating the efficacy of anthelmintics in ruminants (bovine, ovine, caprine). Vet Parasitol 1995 Jun;58(3):181–213.
45. Coles GC, Bauer C, Borgsteede FH, Geerts S, Klei TR, Taylor MA, et al. World Association for the Advancement of Veterinary Parasitology (W.A.A.V.P.) methods for the detection of anthelmintic resistance in nematodes of veterinary importance. Vet Parasitol 1992 Sep;44(1–2):35–44.
46. Forrer A, Khieu V, Schindler C, Schär F, Marti H, Char MC, et al. Ivermectin treatment and sanitation effectively reduce Strongyloides stercoralis infection risk in rural communities in Cambodia. PLoS Negl Trop Dis 2016 Aug;10(8):e0004909.
47. Khieu V, Schär F, Forrer A, Hattendorf J, Marti H, Duong S, et al. High prevalence and spatial distribution of Strongyloides stercoralis in rural Cambodia. PLoS Negl Trop Dis 2014 Jun;8(6):e2854.
48. Croker C, She R. Increase in reports of Strongyloides infection — Los Angeles County, 2013–2014. MMWR Morb Mortal Wkly Rep 2015 Aug 28;64(33):922–3.
49. Keiser PB, Nutman TB. Strongyloides stercoralis in the immunocompromised population. Clin Microbiol Rev 2004 Jan;17(1):208–17.
50. World Health Organization. World Health Assembly adopts resolution on neglected tropical diseases [Internet]. Geneva: World Health Organization; 2013 [accessed 2018 Mar 13]. Available from: http://www.who.int/neglected_diseases/WHA_66_seventh_day_resolution_adopted/en/.