Management of Helicobacter Pylori Infection in Africa: The Challenges and Peculiarities
Vol. 27, Issue 2 (June 2022)
 |
Evaristus S. Chukwudike, MB BCh, FWACP
Consultant Physician and Gastroenterologist, University of Calabar Teaching Hospital
Member, Clinical Research Committee, World Gastroenterology Organisation
Co-Chair, Africa H. pylori Working Group
Mentor and Research Co-Director, African Association of Future Gastroenterologists
Co-Chair, Africa H. pylori Working Group
Calabar, Nigeria
|
 |
|
Steven F. Moss, MD, AGAF, FACG
Professor of Medicine, Brown University
Program Director, Gastroenterology Fellowship Training Program,
Brown University
Director of Endoscopy, Providence VA Medical Center
Providence, Rhode Island, USA
|
|
 |
Akwi W. Asombang, MD, MPH, FACG, FASGE
Interventional Gastroenterologist
Director of Global Health Programs in Gastroenterology
Massachusetts General Hospital
Boston, Massachusetts, USA
|
Helicobacter pylori (H. pylori) is a gram-negative class 1 carcinogen that has chronically infected more than 50% (4.4 billion people) of the world’s population with variable prevalence rates in different populations.1, 2 Africa comprises 54 nations with a population of 1.4 billion people, many of whom have H. pylori infection. Africa has the highest prevalence of H. pylori infection (70.1%) compared to Oceania/Australia with the lowest prevalence of 24.4%.3 Among individual countries, Nigeria in West Africa has the highest global prevalence of 87.7% while Switzerland has the lowest prevalence of 18.9%.3
H. pylori is a major cause of gastroduodenal disease including chronic active gastritis, peptic ulcer disease, mucosa-associated lymphoid tissue (MALT) lymphoma, and gastric cancer. It is an established cause of iron deficiency anemia and idiopathic thrombocytopenia and a suggested cause of many other non-gastroduodenal disorders. While about 75-80% of H. pylori-infected persons do not show clinical symptoms, almost all show evidence of gastritis if biopsies are taken during esophagogastroduodenoscopy. H. pylori gastritis has been designated as an infectious disease with the recommendation of a paradigm shift from treatment of persons with clinical manifestations to the treatment of all infected persons (Kyoto consensus report of 2015).4 As an infectious disease without an available vaccine, the hope of its containment is in the treatment of all infected persons to eradicate the organism and break the chain of transmission driven by the cohort effect.
Eradication of H. pylori is the most effective means to cure and prevent the recurrence of peptic ulcer disease and MALT lymphoma. The risk of gastric cancer is related to the severity and extent of gastric atrophy, intestinal metaplasia, and dysplasia (Correa cascade). H. pylori eradication has been found beneficial in preventing the progression of gastric atrophy and intestinal metaplasia leading to an approximately 50% reduction in the risk of gastric cancer development.5, 6 Globally, there has been a substantial decline in H. pylori eradication rates over the years due to antibiotic resistance necessitating antimicrobial resistance testing as an important tool in H. pylori management.7, 8 H. pylori antimicrobial susceptibility testing is sparse in most parts of Africa. Eradication rates and antibiotic resistance patterns are variable among different geographical regions and largely mirror the type and quantity of antibiotics used in a particular population.9 Eradication failure is more worrisome in Africa already grappling with a huge burden of H. pylori infection prevalence rates occasioned by poor sanitation, over-crowding, low antimicrobial stewardship, poverty, sparse endoscopic services, out-of-pocket payment system of healthcare, and poor medication compliance.10 In a prior study in Africa, H. pylori eradication rates were as low as 30.0%, 44.4%, 50.0%, 53.8%, and 77.8% by protocol analysis and 23.1%, 23.5%, 33.3%, 35.0%, and 41.2% by intention to treat respectively using five different triple therapy combinations.11
H. pylori eradication testing is carried out using a urea breath test, stool antigen test, or endoscopic biopsy specimen. When a urea breath test, stool antigen, or rapid urease test is used, the patient should be off antibiotics, bismuth, and proton pump inhibitor (PPI) at least two weeks before carrying out the test, and a post-treatment test should be carried out at least four weeks after completion of therapy to prevent a false-negative result. An eradication test is not recommended as a primary indication for endoscopy. However, when a biopsy specimen is obtained from other indications for endoscopy, at least two tissue specimens should be taken, each from the antrum and the corpus as H. pylori tend to migrate upwards from the antrum in patients who are receiving PPIs. Histologically, the corpus may look like the antrum in atrophic gastritis and requires that the antral biopsy specimen be separated from that of the corpus. In intestinal metaplasia, multiple gastric biopsy specimens (using Modified Sydney protocol) should be obtained as H. pylori preferentially colonize normal gastric mucosa and do not adhere to the metaplastic gastric mucosa.
If performing H. pylori culture for diagnosis or for antibiotic susceptibility testing, the gastric biopsies should be plated and cultured rapidly, or else placed in a transport medium such as Brucella broth with 20% glycerol and frozen immediately, preferably by a quick freeze at −70 to −80 °C and shipped overnight on dry ice to the laboratory where culture will be performed.8 If maintained in a −70 to −80 °C freezer, the specimen will remain viable for months, if not years. Another transport medium is Portagerm pylori.8 Antimicrobial susceptibility testing using next-generation sequencing (NGS) is an alternative to culture.8 However, the high cost and lack of expertise limit the use of resistance testing in Africa. H. pylori can also be detected in blood by the presence of antibodies, but this serologic test is mainly reserved as a screening test and is not as accurate as the other tests discussed above. Though available and cost-effective, another drawback with using serology to evaluate eradication is that it does not differentiate active from past H. pylori infection.
In Africa, there is marked H. pylori resistance to the most common antimicrobials that are used in combination with PPIs to treat H. pylori infection, namely amoxicillin, clarithromycin, tetracycline, and metronidazole (see Figure 1). Treatment success requires the use of optimized regimens that are defined as consistently achieving high cure rates in adherent patients with susceptible infections. As a general rule, therapeutic regimens that have a ≥90% or, preferably, ≥95% eradication rate locally are recommended and where no available regimen can achieve a ≥90% eradication rate, the most effective regimen(s) available locally should be used.12 Eradication in Africa has not reached the global target of ≥90%.12
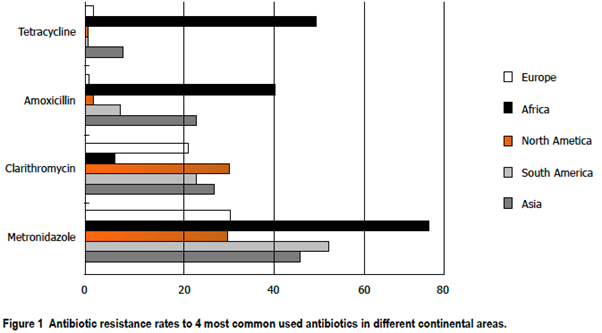
With the exception of clarithromycin, Africa records the highest antibiotic resistance to the common antibiotics (amoxicillin, metronidazole, and tetracycline) used in the treatment of H. pylori infection (Figure 1) which has been attributed to poor antibiotic stewardship. Currently, the suitable common empirical regimens for the treatment of H. pylori include bismuth quadruple therapy, concomitant therapy, sequential therapy, and rifabutin triple therapy.8 Conventional triple-therapy consists of a proton pump inhibitor and two antibiotics: amoxicillin and clarithromycin, or metronidazole and clarithromycin. For use as a first-line regimen, clarithromycin combination triple therapy (PPI, amoxicillin, clarithromycin) is recommended if local clarithromycin resistance is less than 15% (Maastricht V/Florence Consensus report, 2016).14 Metronidazole can replace amoxicillin in patients who are allergic to penicillin. However, high rates of metronidazole resistance in Africa limit its use as a combination for triple therapy. Quadruple therapy should be used as first-line therapy in places with high clarithromycin resistance (>15%).14
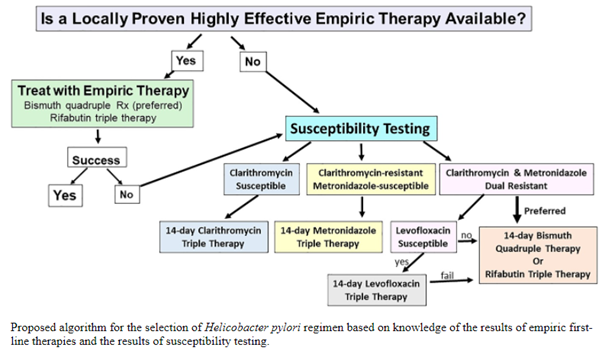
Ideally, treatment of resistant H. pylori infection should prompt antibiotic susceptibility testing (AST- see Figure 2). However, in most parts of Africa where this test is not available, treatment should include the use of quadruple therapy (bismuth-containing quadruple therapy or non-bismuth containing quadruple therapy). Bismuth-containing quadruple therapy consists of bismuth sub-salicylate or sub-citrate administered four times a day, high dose PPI given twice a day, and two antibiotics (metronidazole, tetracycline, amoxicillin, furazolidone or levofloxacin) preferably administered for 14 days. The non-bismuth-containing quadruple (concomitant) therapy is one in which, instead of bismuth, another antibiotic is added to the combination to make it three antibiotics (clarithromycin, amoxicillin, and metronidazole) and a PPI. High-dose PPIs are shown to enhance the efficacy of treatment by raising gastric epithelial pH and working in synergism with antibiotics. Sequential therapy (five days PPI and amoxicillin followed by another five days PPI, metronidazole or tinidazole, and clarithromycin) has not been shown to be more efficacious than quadruple therapy.15
Vonoprazan, a novel type of potassium-competitive acid blocker, provides more rapid and profound acid suppression that is achievable with PPIs. Dual therapy of vonoprazan and amoxicillin or triple therapy of vonoprazan, amoxicillin, and clarithromycin appears highly effective in studies from South East Asia where the drug was first used. Triple therapy of vonoprazan, amoxicillin and rifabutin or levofloxacin may be more effective in some areas in Africa with high clarithromycin resistance. Though vonoprazan is not yet available in Africa, it is hoped it will be available and affordable to help improve the currently low rates of H. pylori eradication in the continent over the next decade.
It is our opinion that the most important methods to improve H. pylori management in Africa would involve creating more awareness of the alarming prevalence of H. pylori infection in the continent, improving antibiotic stewardship, training, and re-training healthcare workers on how to make early diagnoses and appropriate treatment of the infected population as well as scaling up endoscopic services to manage H. pylori-associated complications.
References
1. Cho J, Prashar A, Jones NL, Moss SF. Helicobacter pylori Infection. Gastroenterol Clin North Am. 2021;50(2):261–82.
2. Shah S, Hubscher E, Pelletier C, Jacob R, Vinals L, Yadlapati R. Helicobacter pylori infection treatment in the United States: clinical consequences and costs of eradication treatment failure . Expert Rev Gastroenterol Hepatol. 2022 Apr 1;1–17.
3. Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology. 2017;153(2):420–9.
4. Sugano K, Tack J, Kuipers EJ, Graham DY, El-Omar EM, Miura S, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Vol. 64, Gut. 2015. p. 1353–67.
5. Argueta EA, Moss SF. Management of Helicobacter pylori. Curr Opin Gastroenterol. 2020;36(6):518–24.
6. Roesler BM, Botelho Costa SC, Murilo J, Zeitune R. Eradication Treatment of Helicobacter pylori Infection: Its Importance and Possible Relationship in Preventing the Development of Gastric Cancer. Int Sch Res Netw ISRN Gastroenterol. 2012;9.
7. Savoldi A, Carrara E, Graham DY, Conti M, Tacconelli E. Prevalence of Antibiotic Resistance in Helicobacter pylori: A Systematic Review and Meta-analysis in World Health Organization Regions. Gastroenterology. 2018;155(5):1372-1382.e17.
8. Graham DY, Moss SF. Antimicrobial Susceptibility Testing for Helicobacter pylori Is Now Widely Available: When, How, Why. Am J Gastroenterol. 2022;117(4):524–8.
9. Jaka H, Rhee JA, Östlundh L, Smart L, Peck R, Mueller A, et al. The magnitude of antibiotic resistance to Helicobacter pylori in Africa and identified mutations which confer resistance to antibiotics: systematic review and meta-analysis. BMC Infect Dis 2018 181. 2018;18(1):1–10.
10. Aboderin OA, Abdu AR, Odetoyin BW, Okeke IN, Lawal OO, Ndububa DA, et al. Antibiotic resistance of helicobacter pylori from patients in Ile-Ife, South-west, Nigeria. Afr Health Sci. 2007;7(3):143–7.
11. Solomon O, Ajayi A, Adegun P, Gabriel O, Afolabi O, Solomon O. Effectiveness of Triple Therapy Regimens in the Eradication of Helicobacter pylori in Patients with Uninvestigated Dyspepsia in Ekiti State, Nigeria. Br J Med Med Res. 2015;6(3):278–85.
12. Rimbara E, Fischbach L, Graham D. Optimal therapy for Helicobacter pylori infections. Nat Rev Gastroenterol Hepatol. 2011;8(2):79–88.
13. Ghotaslou R, Leylabadlo HE, Asl YM. Prevalence of antibiotic resistance in Helicobacter pylori: A recent literature review. http://www.wjgnet.com/. 2015;5(3):164–74.
14. Malfertheiner P, Megraud F, ’morain O. Management of Helicobacter pylori infection—the Maastricht V/Florence Consensus Report. Gut. 2017;66:6–30.
15. Gisbert JP, Calvet X. Update on non-bismuth quadruple (concomitant) therapy for eradication of Helicobacter pylori. Clin Exp Gastroenterol. 2012;5(1):23.






