Primary Biliary Cholangitis: New treatment goals and novel salvage therapy
Vol. 28, Issue 1 (March 2023)
 |
Dauris Rosario Lora, MD
Resident Physician, Internal Medicine
Rush University Medical Center
Chicago, Illinois, USA
|
 |
|
Zoë Post, MD
Fellow Physician, Gastroenterology & Hepatology
Rush University Medical Center
Chicago, Illinois, USA
|
|
 |
Nancy S. Reau, MD
Professor, Department of Internal Medicine, Division of Digestive Diseases and Nutrition
Rush University Medical Center
Chicago, Illinois, USA
|
1- Introduction
Primary biliary cholangitis (PBC) is a chronic, autoimmune disease characterized by an inflammatory T-cell mediated destruction against biliary epithelial cells. Studies have pointed to a combination of genetic risk factors and environmental triggers, as part of the etiology of PBC.1,2 It has its highest prevalence in white females, between 40 to 50 years of age, with recent studies showing an unexplained increase in prevalence as of recent years. It is worth noting that prevalence of the disease in other populations besides Caucasians has been hard to estimate given the rarity of the disease. Although it can present asymptomatically, the classical clinical features of PBC include fatigue, pruritus, and, in some cases, xanthelasma. Additionally, patients with PBC are at increased risk for hypercholesterolemia, metabolic bone disease, other autoimmune diseases, cirrhosis, and hepatocellular carcinoma (HCC) in those with cirrhosis. Diagnosis can usually be confirmed by the presence of the anti-mitochondrial antibody, a very specific auto-antibody against multi-enzyme complexes located in the inner mitochondrial membrane, if the usual clinical features of PBC and laboratory abnormalities are present.1,2
Currently, ursodeoxycholic acid (UDCA), and obeticholic acid (OCA) are the only FDA approved treatments for PBC. Weight based UDCA is the current first line therapy, followed by the addition of OCA in those with an inadequate response to UDCA, or intolerance to it. Even with the addition of OCA, there are often cases where patients fail to achieve remission of disease. However, many new promising treatment targets have surfaced.3 Yet, treatment for PBC is currently a very active field of investigation, with various new mechanisms of actions and treatment targets emerging, leading to various clinical trials. In addition, as research points toward better outcomes with more conservative definitions of biochemical remission, treatment guidelines are starting to redefine goals of treatment for PBC patients.4
2- Treatment of PBC: The Present
There are currently two FDA approved treatments for PBC: UDCA and OCA.
2.1 UDCA
UDCA is currently the standard first line therapy for PBC patients and has been demonstrated to significantly impact the course of disease. It is a synthetic bile acid with anti-inflammatory, immunomodulatory, and cytoprotective properties. It has additionally been shown to protect cholangiocytes from cholestatic injury by toxic bile acids, like chenodeoxycholic acid (CDCA). It is started at a dose of 13-15 mg/kg/day, initially divided in two doses though if tolerated it can be given once daily. Higher doses confer the same treatment efficacy with increased adverse events rates.2 Benefits include a reduction in progression to cirrhosis, as well as a decrease in the need for orthotopic liver transplantation (OLT) in PBC patients. It has also been linked to a decrease in LDL levels, development of varices, and slower histologic progression. In fact, patients that are started on UDCA in early stages of the diseases, tend to reach survival rates comparableto the general population. Approximately 40% of patients tend to have suboptimal responses to UDCA therapy which negatively impacts outcomes.2,5
2.2 OCA
OCA was approved by the FDA in May 2016, as a second line drug to be used in combination with UDCA non-responders (UDCA-NR), or as an alternative monotherapy for those who are intolerant to UDCA.2 It is a farnesoid X receptor (FXR) agonist, which leads to suppression of de novo bile acid synthesis and decreased conversion of cholesterol to bile acids. Other mechanisms include anti-inflammatory and anti-fibrotic properties, as well as decreased absorption, secretion, and metabolism of bile acids.2,5
Clinical trials have demonstrated a 25% to 53.9% drop in alkaline phosphatase (ALP) levels in patients started on treatment with OCA in comparison to placebo groups. Reduction in inflammatory markers has also been noted. Additionally, data suggests that the combination of UDCA and OCA could lead to a decrease in the 15-year cumulative incidence of cirrhosis, HCC, OLT need, and death due to liver related complications.2
OCA should be used with caution in patients with cirrhosis. The FDA issued a black box warning in February 2019, given instances of incorrect OCA dosing in cirrhotic patients leading to decompensation, acute liver failure, worsening PBC, and death.2,6 OCA is contraindicated in patients with cirrhosis and evidence of portal hypertension as well as those with decompensated cirrhosis (Child Pugh Turcotte B or C) or a history of prior decompensation.2,6
2.3 Fibrates
Fibrates work by activating the peroxisome proliferator activator receptor (PPAR) alpha. They are traditionally used to treat dyslipidemia. Activation of specifically the α isoform of this receptor, is involved in bile acid synthesis, as well as a decrease of bile acid reuptake by the liver, by inhibitions of transporters. It has also been described to have antithrombotic and anti-inflammatory effects.2,5
Bezafibrate and fenofibrate have been studied as possible treatments in PBC. Fenofibrate is FDA approved for treatment of dyslipidemia, while bezafibrate is not yet approved for use in the US. A meta-analysis by Zhangl et al. shows that both drugs, in combination with UDCA, lead to significant improvement in various disease biomarkers (including ALP, liver enzymes, GGT, albumin levels, etc.) as well as a reduction in the severity of pruritus, without an elevated rate of adverse events. Although fibrates decrease the GLOBE score (Table 1) in PBC patients with suboptimal response to UDCA resistance, survival and rate of OLT seem to be unaffected.7
As previously stated, American Association for the Study of Liver Diseases (AASLD) guidelines recommend fibrates as an alternative to OCA for second line therapy of UDCA-NR. However, they are contraindicated in patients with decompensated liver disease.6
3- Goals of Therapy in PBC
The first line of treatment for PBC is a trial of weight-based (13 mg/kg/day in divided doses) UDCA therapy. Treatment response to UDCA is assessed utilizing serum ALP and total bilirubin (TB) as surrogates for disease activity. Response is usually evaluated after one year of treatment with UDCA, using any of the response criteria described in Table 1. Besides being a UDCA-NR, alternative indications for the addition of a second agent include high risk for progression of disease or evidence of hepatic fibrosis and/or progression to cirrhosis. In those with the above indications, guidelines currently recommend the addition of 5 mg OCA with titration to 10 mg if the patient had adequate toleration but suboptimal response to OCA. Fibrates have recently emerged as an alternative second line of treatment. If failure to reach treatment goals still persists after addition of either of these agents, either OLT or clinical trials are considered. Given that both fibrates and OCA are contraindicated in patients with decompensated cirrhosis, these patients currently lack an established option for second line therapy, besides OTC.1,2,6 Table 2 summarizes the AASLD guideline statements for treatment of PBC.
|
Table 1. Scoring systems for assessment of response to UDCA
|
|
Name
|
Response Criteria
|
|
GLOBE (8)
|
Bilirubin, ALP, albumin and platelet count 12 months. Age at baseline.
|
|
Paris-I (9)
|
ALP > 3 X ULN or AST > 2 X ULN or bilirubin > 1.0
|
|
Paris-II (10)
|
All three of the following: ALP > 1.5 X ULN, AST 1.5 X ULN, bilirubin >1 mg/dl after 1 year on UDCA
|
|
Rotterdam (11)
|
Bilirubin > 1 X ULN and/or albumin < 1 x ULN
|
|
Barcelona (12)
|
Decrease in ALP < 40% and ALP > 1.0 x ULN
|
|
Toronto (13)
|
ALP > 1.67 x ULN after 2 years of UDCA
|
|
Rochester (14)
|
ALP > 2 X ULN
|
Alkaline phosphatase, ALP; upper limit of normal, ULN; aspartate aminotransferase, AST; UDCA, ursodeoxycholic acid
|
Table 2. AASLD Guideline Recommendations for Treatment of PBC2, 6
|
|
- “UDCA in a dose of 13 to 15 mg/kg/day orally is recommended for patients with PBC who have abnormal liver enzyme values regardless of histologic stage.”
- “Biochemical response to UDCA should be evaluated at 12 months after treatment initiation to determine whether patients should be considered for second-line therapy”
- “Patients who are inadequate responders to UDCA (Table 1) should be considered for treatment with OCA, starting at 5 mg/day.”
- “Fibrates can be considered as off-label alternatives for patients with PBC and inadequate response to ursodeoxycholic acid, although fibrates are discouraged in patients with decompensated liver disease.”
- “OCA is contraindicated in patients with advanced cirrhosis. This is defined as cirrhosis with current or prior evidence of liver decompensation (e.g., encephalopathy, coagulopathy) or portal hypertension (e.g., ascites, gastroesophageal varices, or persistent thrombocytopenia). Furthermore, we would recommend careful monitoring of any patient with cirrhosis, even if not advanced, receiving OCA.”
|
|
| |
| |
| |
UDCA, ursodeoxycholic acid; PBC, primary biliary cholangitis; OCA, obeticholic acid
3.1 Predictive Models
Various predictive models exist and are used as tools to assess the prognosis in patients treated with UDCA. The most widely used, and validated predictive model, is the GLOBE score, which is based on data collected from the Global PBC Study group, a multicenter, international study in 15 liver centers, from eight different countries across Europe and North America, comprising 4,500 PBC patients. The GLOBE score predicts the risk of OLT or death a year after initiation of treatment, using factors obtained at the time of initiating UDCA including age, TB levels, serum ALP levels, platelet count, and albumin.8
Multiple other models have been designed to assess the response of patients to UDCA.1 Table 1 summarizes the most widely used models, as well as each of their response criteria. There is currently no agreement on which criteria is the best for defining a UDCA-NR. In general, most include ALP levels, given the existing data supporting its role as a surrogate for response to treatment.1 It should be noted that normalization of ALP is not a criterion for response, with the Paris-II model having the lowest ALP threshold (1.5 times the upper limit of normal) of all the current models designed.1,2,10 Clinical guidelines by the AASLD recommend the use of these criteria, but do not show any preference of one over the other.2
4. Future Goals of Therapy: Are Normalized ALP and Bilirubin Levels the New Goal?
Recent studies point towards normal ALP levels as the desired biochemical goal for effective PBC therapy. Jones et al. demonstrated that PBC patients with any abnormal levels of ALP had significantly higher levels of various inflammatory markers in comparison to those with normalized liver biochemistry, suggesting ongoing disease activity even in what would be considered a “responder” by most criteria.15 Additionally, an analysis made with data extracted from the GLOBAL PBC Study Group, compared ten-year survival rates of a cohort of PBC patients and found an association between normal ALP levels and lower risk for OLT or death in these patients.4
Normal TB levels have also been correlated with better outcomes in PBC patients. The above-mentioned analysis on data from the GLOBAL PBC Study Group demonstrated that TB levels below 0.6 x ULN were correlated with an improvement in 10-year survival, and a decreased risk of OLT or death, in comparison to those with TB levels > 0.6 X ULN (Figure 1). Above these levels, a linear relationship was demonstrated between TB and the risk for OLT or death (Figure 1). Interestingly enough, patients with TB >0.6 x ULN and normal ALP levels had similar survival rates to those with TB levels < 0.6 x ULN.4 Similarly, Jones et al. found that 88% of those patients with normal ALP levels, which as mentioned above, was correlated with decreased disease activity, presented TB levels < 0.6 x ULN.15
Figure 1. Survival estimates in patients with normal TB (stratified by 0.6 X ULN threshold) and abnormal bilirubin.4
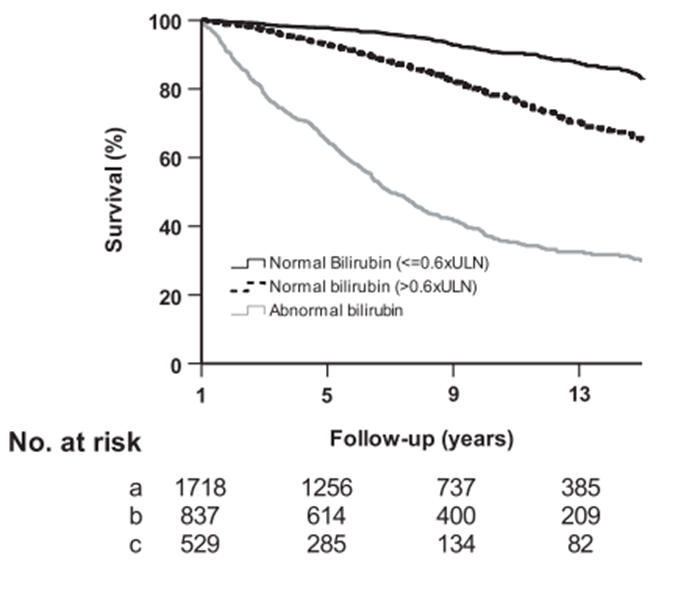
Upper limit of normal, ULN
Given that, as discussed above, none of the existing criteria really prioritize normalization of ALP or TB levels to qualify a successful treatment, it is easy to see how findings like this could have very important implications on the future management of PBC patients, particularly when deciding to start any treatment beyond first line therapy. These results open the door to a revision on what is the current criteria for exploring treatment beyond UDCA, especially now that treatment options for PBC are in rapid development, with multiple promising options of salvage therapy currently under investigation.
Finally, an argument could be made for when exactly we should assess treatment response to UDCA. Most of the predictive models described above set 12 months of treatment as the “checkpoint” for assessing appropriate response in therapy. However, it seems that around 90% of the improvement in those who respond to UDCA is seen within six to nine months of treatment.16 This leads us to question if an earlier assessment of response to treatment would be more ideal.
5- Treatment of PBC: The Future
5.1 Investigational Fibrates
Seladelpar is a PPAR-δ agonist, a receptor found in hepatocytes, cholangiocytes and various immune cells known to play a role in the pathogenesis of PBC. Its mechanism is thought to consist in a reduction of bile acid synthesis as well as an increase in lipid degradation. It has also been found to show anti-inflammatory and anti-fibrotic activity.5,17 ENHANCE 3 studied the efficacy of seladelpar in UDCA-NR. 240 patients were distributed into three treatment arms: seladelpar 5 mg, 10 mg, and placebo. 78.2% of patients in the 10 mg arm, and 57.1% achieved a 15% or greater decrease in ALP, and a TB < ULN. 27.3% of the patients in 10 mg arm achieved normalization of the ALP levels.17
5.2. FGF-19 Analogues
Fibroblast growth factor-19 (FGF-19) is another novel treatment target for PBC. FGF-19 is regulated by the FXR, and it acts to decrease the synthesis of bile acids, by downregulation of genetic expression of the rate-limiting enzyme cholesterol-7α–hydroxylase. NGM282 is an analogue of FGF19. In a randomized placebo-controlled trial by Mayo et al., daily administration of either 0.3 mg or 3mg NGM282 demonstrated significant reductions in ALP, AST, ALT and GGT levels in UDCA-NR. At least 50% of the patients taking the experimental drug experienced at the very least a 15% reduction from their baseline ALP level. No difference was observed regarding TB levels between the NGM282 and the placebo group. The drug was also generally well tolerated, with minimal side effects.18
3.5. Non-Bile Acid FXR Agonists
New FXR agonists, without a bile acid like structure, are currently under investigation for their utility for treating PBC. These include tropixefor, cilofexor, and EDP-305.
Schramm et al. published results of their randomized clinical trial of tropixefor vs. placebo in patients with PBC with suboptimal response to UDCA. Patients were randomized to receive either 30, 60, 90, or 150 μg of tropixefor once daily for 28 days. Primary endpoints included safety, and reduction in various liver biomarkers. Most adverse events were mild, with pruritus being the most common. At day 28, ALP levels were seen to be significantly reduced from baseline in the 60 and 90 μg groups (33.3-41.7%), however none of the patients under treatment achieved normalization of their ALP levels, and very few achieved a decrease below 1.67 x ULN. There was a 26-72% decrease in GGT from baseline seen across all doses, with a significant portion achieving normal levels. No significant differences between TB levels were seen between the study and placebo group.19
A phase two randomized, placebo-controlled clinical trial involving the non-steroidal FXR agonist, cilofexor, demonstrated that in PBC patients who were UDCA-NR, 100 mg of cilofexor, for 12 weeks, led to a significant median reduction of serum ALP at (13.8% p= 0.005 vs placebo), GGT (47.7%; p<0.001), C-reactive protein (CRP; 33.6%, p=0.03) and primary bile acids (30.5%, p=0.008). Minimal side effects were observed.20
EDP-305 is both a steroid and non-steroid FXR agonist. Results from the INTREPID study (NCT03394924) set out to demonstrate its safety and efficacy in PBC patients with no response to UDCA. A total of 68 subjects were randomized to either placebo, 1 mg or 2.5 mg of EDP-305 for 42 weeks. An ALP reduction of at least 45% was noted in both groups receiving the drug, compared to an 11% in the placebo group. Pruritus was the most common side effect noted.21
5.3. Immunomodulators
Given the principal role of innate immune dysregulation in the pathogenesis of PBC, it has been hypothesized that immunomodulators could play a significant role in its treatment. Thus, various immunomodulators have been tested in UDCA-NR patients. However, many of these drugs have not been found to be of any substantial benefit. This includes drugs such as budesonide, rituximab, ustekimumab, azathioprine, mycophenolate, methotrexate, and colchicine.2,5
Mesenchymal stem cells (MSC) transplants have been a topic of interest in the treatment of end stage PBC patients as a possible alternative to liver OLT. So far, although clinical trials have been very few, they have shown promising results including improvement in disease biomarkers, relief of extrahepatic symptoms and improvement and/or stabilization of disease by liver histology. As a result, there are ongoing trials with bigger patient samples dedicated to investigating the safety and efficacy of MSC transplants in UDCA-NR patients.5,22
6- Conclusion
The treatment and management of PBC patients is evolving. Although criteria exists to determine the response of treatment and need for second line therapies, data suggests that normalization of ALP and bilirubin may be more important therapeutic targets. Findings demonstrate that even acceptable levels of ALP in PBC patients still may correlate with a certain degree of disease activity. These findings carry even more weight when we look at the high number of promising therapeutic options, with many different mechanisms of action, currently being investigated. Although data is still limited in many of the novel PBC treatments mentioned above, early clinical trials have shown remarkable potential. There is a lot of promise for a future in which we will have multiple safe and efficacious treatment options available, that could help us reach the goal of normalizing serum biochemistries and achieving a complete remission or minimal progression of the disease.
References
1. Younossi ZM, Bernstein D, Shiffman ML, Kwo P, Kim WR, Kowdley KV, Jacobson IM. Diagnosis and Management of Primary Biliary Cholangitis. Am J Gastroenterol. 2019 Jan;114(1):48-63. doi: 10.1038/s41395-018-0390-3. PMID: 30429590.
2. Lindor, K.D., Bowlus, C.L., Boyer, J., Levy, C. and Mayo, M. (2019), Primary Biliary Cholangitis: 2018 Practice Guidance from the American Association for the Study of Liver Diseases. Hepatology, 69: 394-419. https://doi.org/10.1002/hep.30145
3. Aguilar MT, Chascsa DM. Update on Emerging Treatment Options for Primary Biliary Cholangitis. Hepat Med. 2020 May 25;12:69-77. doi: 10.2147/HMER.S205431. PMID: 32547264; PMCID: PMC7259454.
4. Murillo Perez CF, Harms MH, Lindor KD, van Buuren HR, Hirschfield GM, Corpechot C, van der Meer AJ, Feld JJ, Gulamhusein A, Lammers WJ, Ponsioen CY, Carbone M, Mason AL, Mayo MJ, Invernizzi P, Battezzati PM, Floreani A, Lleo A, Nevens F, Kowdley KV, Bruns T, Dalekos GN, Gatselis NK, Thorburn D, Trivedi PJ, Verhelst X, Parés A, Janssen HLA, Hansen BE; GLOBAL PBC Study Group. Goals of Treatment for Improved Survival in Primary Biliary Cholangitis: Treatment Target Should Be Bilirubin Within the Normal Range and Normalization of Alkaline Phosphatase. Am J Gastroenterol. 2020 Jul;115(7):1066-1074. doi: 10.14309/ajg.0000000000000557. PMID: 32618657.
5. Floreani A, Gabbia D, De Martin S. Update on the Pharmacological Treatment of Primary Biliary Cholangitis. Biomedicines. 2022 Aug 20;10(8):2033. doi: 10.3390/biomedicines10082033. PMID: 36009580; PMCID: PMC9405864.
6. Lindor KD, Bowlus CL, Boyer J, Levy C, Mayo M. Primary biliary cholangitis: 2021 practice guidance update from the American Association for the Study of Liver Diseases. Hepatology. 2022;75:1012–1013. https://doi.org/10.1002/hep.32117
7. Zhang H, Li S, Feng Y, Zhang Q, Xie B. Efficacy of fibrates in the treatment of primary biliary cholangitis: a meta-analysis. Clin Exp Med. 2022 Nov 1. doi: 10.1007/s10238-022-00904-2. Epub ahead of print. PMID: 36318376.
8. Lammers WJ, Hirschfield GM, Corpechot C, et al. Development and validation of a scoring system to predict outcomes of patients with primary biliary cirrhosis receiving ursodeoxycholic acid therapy. Gastroenterology. 2015;149:1804–12
9. Corpechot C, Abenavoli L, Rabahi N, Chretien Y, Andreani T, Johanet C, et al. Biochemical response to ursodeoxycho-lic acid and long-term prognosis in primary biliary cirrhosis. Hepatology. 2008;48:871-877
10. Corpechot C, Chazouilleres O, Poupon R. Early primary bil-iary cirrhosis: biochemical response to treatment and predic-tion of long-term outcome. J Hepatol. 2011;55:1361-1367.
11. Kuiper EM, Hansen BE, de Vries RA, den Ouden-Muller JW, van Ditzhuijsen TJ, Haagsma EB, et al. Improved prog-nosis of patients with primary biliary cirrhosis that have a bio-chemical response to ursodeoxycholic acid. Gastroenterology. 2009;136:1281-1287.
12. Pares A, Caballeria L, Rodes J. Excellent long-term survival in patients with primary biliary cirrhosis and biochemical response to ursodeoxycholic Acid. Gastroenterology. 2006;130:715-720.
13. Kumagi T, Guindi M, Fischer SE, Arenovich T, Abdalian R, Coltescu C, et al. Baseline ductopenia and treatment response predict long-term histological progression in primary biliary cirrhosis. Am J Gastroenterol. 2010;105:2186-2194.
14. Angulo P, Lindor KD, Therneau TM, Jorgensen RA, Malinchoc M, Kamath PS, et al. Utilization of the Mayo risk score in patients with primary biliary cirrhosis receiving ur-sodeoxycholic acid. Liver. 1999;19:115-121.
15. Jones DEJ, Wetten A, Barron-Millar B, Ogle L, Mells G, Flack S, Sandford R, Kirby J, Palmer J, Brotherston S, Jopson L, Brain J, Smith GR, Rushton S, Jones R, Rushbrook S, Thorburn D, Ryder SD, Hirschfield G; UK-PBC Research Consortium, Dyson JK. The relationship between disease activity and UDCA response criteria in primary biliary cholangitis: A cohort study. EBioMedicine. 2022 Jun;80:104068. doi: 10.1016/j.ebiom.2022.104068. Epub 2022 May 21. PMID: 35609437; PMCID: PMC9130524. 16. Jorgensen RA, Dickson ER, Hofmann AF, Rossi SS, Lindor KD. Characterisation of patients with a complete biochemical response to ursodeoxycholic acid. Gut. 1995;36:935-938.
17. ENHANCE: Safety and Efficacy of Seladelpar in Patients With Primary Biliary Cholangitis-A Phase 3, International, Randomized, Placebo-Controlled Study. Gastroenterol Hepatol (N Y). 2021 Feb;17(2 Suppl 3):5-6. PMID: 34135711; PMCID: PMC8191827.
18. Mayo MJ, Wigg AJ, Leggett BA, Arnold H, Thompson AJ, Weltman M, Carey EJ, Muir AJ, Ling L, Rossi SJ, DePaoli AM. NGM282 for Treatment of Patients With Primary Biliary Cholangitis: A Multicenter, Randomized, Double-Blind, Placebo-Controlled Trial. Hepatol Commun. 2018 Aug 30;2(9):1037-1050. doi: 10.1002/hep4.1209. PMID: 30202819; PMCID: PMC6128239.
19. Schramm C, Wedemeyer H, Mason A, Hirschfield GM, Levy C, Kowdley KV, Milkiewicz P, Janczewska E, Malova ES, Sanni J, Koo P, Chen J, Choudhury S, Klickstein LB, Badman MK, Jones D. Farnesoid X receptor agonist tropifexor attenuates cholestasis in a randomized trial in patients with primary biliary cholangitis. JHEP Rep. 2022 Jul 21;4(11):100544. doi: 10.1016/j.jhepr.2022.100544. PMID: 36267872; PMCID: PMC9576902.
20. Kowdley KV, Minuk GY, Pagadala MR, Gulamhusein A, Swain MG, Neff GW, et al. The Nonsteroidal Farnesoid X Receptor (FXR) Agonist Cilofexor Improves Liver Biochemistry in Patients with Primary Biliary Cholangitis (PBC): A Phase 2, Randomized, Placebo-Controlled Trial [Abstract 45]. Hepatology AASLD Abstracts 2019;70 (Suppl 1):31A-2A.
21. Kowdley, KV., Bonder, A., Heneghan, MA., Hodge, AD., Ryder, SD., Sanchez, AJ. Vargas, V., Zeuzem, S., Ahmad, A., Larson, K., et al. Final data of the phase 2a INTREPID study with EDP-305, a non-bile acid farnesoid X receptor (FXR) agonist. Hepatology 2020, 72, 131–1159
22. Yang Y, Zhao RC, Zhang F. Potential mesenchymal stem cell therapeutics for treating primary biliary cholangitis: advances, challenges, and perspectives. Front Cell Dev Biol. 2022 Jul 18;10:933565. doi: 10.3389/fcell.2022.933565.





