Quality in Diagnostic Upper Gastrointestinal Endoscopy
Vol. 28, Issue 2 (June 2023)
 |
Gary Morrison, MB BCh BAO, MRCP
Specialty Registrar, Northern Ireland Medical and Training Agency
Northern Ireland
|
 |
|
Tony C Tham, MD, MSc, FRCP, FESGE
Consultant Gastroenterologist, Ulster Hospital
Dundonald, Belfast, Northern Ireland
|
|
Glossary of Acronyms/Abbreviations:
AGA - American Gastroenterological Association
AI - Artificial Intelligence
BO - Barrett's Oesophagus
BSG - British Society of Gastroenterology
CAD - Computer Aided Detection
ESGE - European Society of Gastrointestinal Endoscopy
JAG - Joint Advisory Group of GI Endoscopy
JETS - JAG Endoscopy Training System
IM - Intestinal Metaplasia
NBI - Narrow Band Imaging
SCC - Squamous Cell Carcinoma
UGI - Upper Gastrointestinal
WLE - White Light Endoscopy
Introduction
With the rising incidence of upper gastrointestinal malignancy worldwide, oesophagogastroduodenoscopy (OGD/EGD) has become an ever more vital tool in both the diagnostics and therapeutics of these malignancies. In the western world the incidence of oesophageal adenocarcinoma is dramatically increasing, leading to an increased demand for OGD by over 50% in the United States since the turn of the millennium.1 Considering the precursor lesions, Barrett’s oesophagus and gastric intestinal metaplasia are both endoscopically detectable and treatable, this figure is understandable. Likewise, in high-risk regions for gastric cancer such as Japan and South Korea, OGD has become a first line screening modality. The cancer “miss rates” defined as upper GI cancers diagnosed following a normal OGD in the three years prior to diagnosis is approximately 11.3%.2 Paying attention to key performance indicators, i.e. the quality of the procedure, when performing OGD may be able to reduce this miss rate. In this article, we summarise the key recommendations from available worldwide guidance from societies including, AGA, ESGE, BSG and an Asian consensus guideline. Key performance indicators of diagnostic OGD will be assessed and the impacts this will have in detection and treatment of upper GI cancers and their precursors.
Pre procedure
Adequate preparation prior to endoscopy optimises conditions for a high quality study, with sufficient patient comfort and mucosal views to attain a satisfactory diagnostic OGD. The guidances recommend appropriate indications for the procedure, assessment of fitness and consent prior to procedure, as well as a fasting protocol. ESGE advises two hours fasting for liquids and six hours for solids. Both AGA and BSG highlight the importance of the competency of the endoscopist, with the latter referencing JAG/JET accreditation and minimum procedure rate of 100 procedures per year following obtainment of sign off.3
Sedation
No studies to date have directly assessed the role of sedation on the endoscopic detection rate of superficial upper GI neoplasia. However, one large placebo-controlled randomised controlled trial showed that midazolam use increased patient cooperation, satisfaction and willingness to repeat procedure.2 This has the possible potential to result in higher compliance and uptake with future screening, therefore influencing the increase in detection of upper gastrointestinal malignancies. There is a paucity of evidence as to an increased risk of complications in routine clinical practice, however, JETS/JAG recommend no more than 5mg Midazolam or 100mcg Fentanyl in under 70-year-old patients and no more than 2mg Midazolam or 50mcg Fentanyl for those patients over 70 years of age.3, 4
Documentation of procedure duration
The consensus of a seven-minute minimum diagnostic UGI endoscopy procedure is widely agreed while the Asian consensus recommends a minimum eight-minute procedure. Timing commences following intubation of the oesophagus and finishing at the point of extubation. Studies have shown that endoscopists with an average procedure time of at least seven minutes are up to three times more likely to identify dysplastic lesions and gastric cancers compared with those taking an average of less than seven minutes.5 This highlights the importance of close inspection, and in certain circumstances an even more prolonged inspection time is advocated, such as Barrett’s and gastrointestinal metaplasia surveillance. In Barrett’s surveillance there is some evidence that a “Barrett’s inspection time” of >1 min/cm is associated with a significantly greater detection of high-grade dysplasia and adenocarcinoma. Barrett’s oesophagus may be present in up to 5 to 15% of high-risk patients.6 This should be documented as per Prague classification, a validated, widely used protocol describing both the circumferential and maximal extent of the affected area. Barrett’s segment lesions should be documented and described as per Paris classification, photographed and biopsied. The Seattle protocol should be followed as recommended by the BSG, ESGE and AGA.
Accurate photodocumentation
A meta-analysis showed that 11.3% of upper GI cancers were missed during OGDs performed up to 36 months before diagnosis.2 With rising incidence of gastric cardia cancers the J manoeuvre is an important aspect of OGD. It is suggested that endoscopic photo mapping could help mitigate the risk of missing cancers in these locations. All landmarks of interest should be visualised from the oesophageal sphincter to the second part of the duodenum.7 The BSG advises a minimum of eight photographs of the anatomical landmarks; upper oesophagus, gastro oesophageal junction, fundus in retroflexion, stomach body, incisura, antrum, duodenal bulb and second part of duodenum. ESGE advises ten images with the addition of proximal oesophagus, upper end of gastric folds and major papilla. See below image.4,6 The Asian consensus guideline goes further again, mentioning two studies proposing a systematic screening protocol of 20-22 photographs of the stomach.2 However, there is no well-designed study comparing the diagnostic yield of increased photographic mapping.
Photographs of landmarks (Image 1)
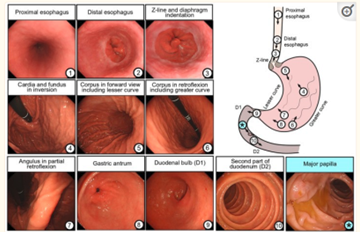
Standardised terminology
ESGE maintains that the quality of the endoscopy is inextricably linked to the quality of the report and strongly recommend that abnormal findings should be reported according to internationally validated and standardised terminology.6 Such examples include use of the Paris Classification in describing early neoplastic lesions and the Prague classification for Barrett’s oesophagus, which have already been referenced. The Los Angeles classification for describing erosive reflux esophagitis has been in use since 1996 and again demonstrates a relatively straightforward and reproducible scoring system. Table 1 is a non-exhaustive list of some of the common internationally validated classification systems. Scoring systems in endoscopy allow better communication between physicians and researchers, as well as being especially important in cases of international collaboration.1
Table of common classification systems (Table 1)
|
Location
|
Condition
|
Classification
|
|
General
|
Neoplastic Lesions
|
Paris
|
|
Oesphagus
|
Barrett’s
|
Prague
|
|
|
Erosive Oesophagitis
|
Los Angeles (LA)
|
|
|
Caustic Oesphagitis
|
Zargar
|
|
|
Varices
|
Baveno
|
|
Stomach
|
Bleeding Ulcers
|
Forrest
|
|
Duodenum
|
Adenomas in patients with FAP
|
Spigelman
|
(1,8)
Biopsy protocols
As per the aforementioned Seattle protocol, all the leading gastroenterology societies advocate the use of four quadrantic biopsies for every 2cm of Barrett’s oesophagus, as well as targeting concerning lesions for early detection of dysplasia and neoplasia. Other such biopsy protocols include the use of the Sydney protocol for surveillance of gastric intestinal metaplasia and atrophic gastritis. BSG advocates two no-targeted samples from the gastric antrum, two from the gastric body and one from the incisura. The BSG advises that cases of iron deficiency anaemia should have gastric antral and body biopsies to exclude gastric atrophy.4 Accurate diagnosis of eosinophilic oesophagitis recommends six biopsies from at least two different areas of the oesophagus, usually the proximal and distal or mid-oesophagus. This should be performed in >90% of dysphagia and food bolus presentations, according to the BSG position statement in 2017. Cases of LA classification C and D should result in biopsies and repeat endoscopy at six to eight weeks to ensure healing and exclude malignancy. Likewise, cases of gastric ulcers should be described and biopsied with repeat OGD in six to eight weeks. Gastric and duodenal ulcers should prompt H. pylori testing and eradication in the event of a positive result.4 Biopsies from the duodenum are required for the diagnosis of celiac disease in adults. In cases where celiac is suspected, such as iron deficiency anaemia, weight loss and diarrhea, four biopsies from the duodenum should be taken.1, 4
Polyps and malignant lesions
The presence of gastric polyps should be recorded with the number, size, location and morphology described, and representative biopsies taken.1 In the event of identifying a malignant lesion these should be documented as per anatomical location, number, size and morphology as well as any abnormalities of the background mucosa. A minimum of six biopsies should be obtained. Biopsies should be collected prior to dilatation of any stricture, due to the small risk of converting localised tumour into disseminated disease via perforation.4
Image enhancing techniques and CAD
Various imaging techniques have been developed in recent times to assist in the recognition of lesions in the GI tract. The three different organs assessed during OGD makes for a wider spectrum of epithelia and therefore pathology, when compared to colonoscopy.1 Such imaging techniques include chromoendoscopy with Lugol staining in the oesophagus, which has been shown to improve detection of squamous neoplasia. Both the BSG and ESGE recommend its use where SCC is suspected.1, 4, 6 Other techniques include narrow spectrum imaging such as narrow band imaging (NBI). A multicentre RCT reported that detection of Gastric Intestinal Metaplasia was significantly higher using NBI compared with white light endoscopy (WLE).2 In recent years there have been significant advancements in the use of artificial intelligence (AI) and computer aided detection (CAD) which could increase the diagnostic yield of pre-malignant or early malignant lesions.1 For now, these remain outside of the current guidance.
Reporting systems, Audit and Complication documentation
All procedures should be documented using reporting systems where complications can be documented and audited. ESGE advises these should be electronic and have structured data entry, limiting free text that are not searchable. Reporting systems should include information such as patient satisfaction, adverse events, and surveillance recommendations.1, 6 After OGD readmission, mortality and complications should be audited, with units also auditing the rates of missed cancers, defined as malignancy identified within three years of OGD.
Conclusion
OGD remains an operator dependent procedure and as incidence of UGI malignancies and their precursor lesions are on the rise, quality in the performance of gastroscopy is paramount. Just as detection of malignancy (especially early) and premalignant lesions is important, the documentation of benign pathologies where monitoring guides treatment and surveillance is equally important. The international societies display slight variation in the minimum standards, but the themes are similar, such as high quality examination and reporting with use of photodocumentation, adequate inspection time and use of the readily available classification systems. While there is a paucity of evidence towards some measures increasing the yield of neoplasia detection, such as use of sedation, all concur that a minimum procedure time of seven minutes increases diagnostic yield significantly. By striving to improve quality in OGD, variation in practice could be reduced and performance standards increased for individual endoscopists and units around the world.
References:
- Quality indicators in diagnostic upper gastrointestinal endoscopy; Therap Adv Gastroenterol, Januszewicz and Kaminski, 2020
- An Asian consensus on standards of diagnostic upper endoscopy for neoplasia; Gut, Wai Yan Chiu et al., 2018
- JAG accreditation programme Guide to meeting the quality and safety standards; JAG, 2019
- Quality standards in upper gastrointestinal endoscopy; BSG, Beg et al., 2017
- Longer examination time improves detection of gastric cancer during diagnostic upper gastrointestinal endoscopy; Clin Gastroenterol Hepatol, Teh JL et al., 2015
- Performance measures for upper GI endoscopy; ESGE, Bisschops et al.
- Quality Indicators for EGD; American Journal of Gastroenterology, Park et al., 2015
- Minimal standard terminology for gastrointestinal endoscopy WEO (formerly known as OMED); Committee for standardization and terminology, Lars Aabakken et al., 2008




