The Future of Gastroenterology
Vol. 28, Issue 4 (December 2023)
 |
José C. Marín-Gabriel, MD
Endoscopy Unit, Department of Gastroenterology
Associate Professor
University Hospital “12 de Octubre”,
Complutense University of Madrid
Madrid, Spain
|
 |
Julia Arribas, MD
Endoscopy Unit, Department of Gastroenterology
University Hospital “12 de Octubre”
Madrid, Spain |
|
 |
María Moreno-Sánchez, MD
Endoscopy Unit, Department of Gastroenterology
University Hospital “12 de Octubre”
Madrid, Spain |
INTRODUCTION
Gastroenterology has experienced significant advances in recent decades, transforming the diagnosis and treatment of many gastrointestinal diseases. From the development of flexible endoscopy in the 1960s to the recent advancements in artificial intelligence, technology continues to drive our specialty forward.1
Predicting the future always proves to be a complex exercise. Precision medicine will likely play an increasingly important role, enabling personalized therapies based on genetic, molecular, or immunologic profiles.2 Furthermore, advancements in artificial intelligence and machine learning are already showing their potential to enhance lesion detection, screening, and diagnosis.3
Since attempting to encompass all possible aspects related to the future of the specialty is not a realistic goal, we will focus on the following topics: telemedicine, artificial intelligence in endoscopy, and third-space endoscopy.
TELEMEDICINE
Telemedicine has emerged as a valuable tool for providing gastroenterology care, especially following the COVID-19 pandemic. Virtual consultations offer a convenient and efficient way to conduct follow-up visits and manage chronic diseases, allowing for more frequent and convenient monitoring of patients. It also facilitates endoscopic surveillance after colorectal cancer surgery, increasing adherence to recommendations by 20%.4 Virtual consultations can even replace in-person visits to address patient queries prior to colonoscopies, and could be used for genetic counseling as well. In the latter case, however, a study showed that healthcare professionals preferred non-face-to-face visits only to deliver negative results from genetic tests.5 Nevertheless, studies suggest high levels of satisfaction among patients and providers.6
However, several considerations must be addressed. Patient data privacy and security are paramount, and appropriate protections for health data must be established. Equity is also a concern, as high-speed internet access and the need for electronic devices are not uniformly distributed across all countries or social strata. Patients from rural areas, the elderly, or those with low resources may be excluded if these disparities are not addressed.7
Furthermore, certain procedures and conditions may not be suitable for telemedicine. More research is needed to determine the accuracy of virtual diagnosis and management compared to in-person care. However, ultimately, this technology presents an opportunity to improve access and reduce costs, provided it is carefully implemented with assurances of quality and equity.8
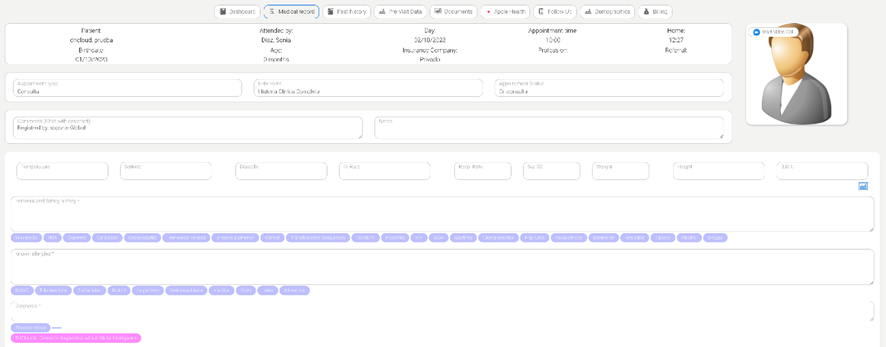
Finally, artificial intelligence (AI) systems, which are based on natural language models, are already being integrated into electronic health records and are expected to further enhance the efficiency of video consultations.9 Among their functions are suggesting diagnoses, recommending complementary tests, and even evaluating potential interactions between different treatments (Figure 1).
Figure 1- An artificial intelligence system integrated into electronic health records (DriCloud, Massive Bionics L.L.C.). By clicking the pink button below the “Diagnosis” field, various diagnostic suggestions can be generated by artificial intelligence based on the patient's medical history and physical examination.
ARTIFICIAL INTELLIGENCE IN ENDOSCOPY
Artificial intelligence (AI), especially deep learning (DL) techniques, enables machines to analyze vast amounts of images used for training and extract specific features. This capability allows the system to diagnose newly acquired clinical images. One of the most widely used network models in this field is convolutional neural networks.10
Currently, the main focuses of AI in endoscopy are cancer detection, identifying the depth of cancer invasion, and detecting and classifying different lesions based on their pathological diagnosis. In gastric cancer detection, AI systems have demonstrated high accuracy. Two recently published meta-analyses showed an overall AI pooled sensitivity for gastric cancer detection above 92%, with a specificity of 88%.11 These figures are difficult to achieve for most community-based endoscopists.
In Barrett's disease (BE), the overall sensitivity and specificity for detecting BE-associated tumors are approximately 90%. Importantly, some studies have demonstrated that DL can accurately detect focal lesions by developing the system not only on still images but also in real-time diagnosis.12
Selecting patients for endoscopic resection of gastrointestinal cancer crucially depends on determining the depth of tumor invasion. Although extensive research is ongoing, AI systems are not ready for this purpose. Recently, Bang et al. developed a DL model using upper gastrointestinal endoscopy images to classify the depth of invasion in gastric tumors (mucosal restriction and submucosal invasion) with 77.3% accuracy in external test sets.13
Another essential use of AI systems is recognizing different anatomic locations. This capacity could help endoscopists follow quality measures and ensure that all locations have been appropriately examined. By using a real-time system to detect blind spots named ENDOANGEL, Wu et al. conducted a five-center Randomized Controlled Trial (RCT) which found that the number of blind spots in the ENDOANGEL assisted group was significantly lower than in the non-assisted group.14
In the field of colonoscopy, AI was thought to improve the adenoma detection rate by aiding in the detection of polyps. Initially, several patient-based prospective studies were published, and in recent years, multiple RCTs have proved that computer-aided detection colonoscopy (CADe) had a significantly higher ADR compared to standard colonoscopy.15 However, this advantage has been mainly proven for lesions smaller than one cm. Repici et al. showed that the smaller the adenoma, the greater the ADR advantage of the CADe system over humans, regardless of polyp shape or location.16
AI has also been applied to capsule endoscopy (CE). Automated detection of bleeding sites, ulcers, tumors, and various small bowel diseases is under investigation. A recent meta-analysis found that CE had an overall aggregate accuracy of 95.4% in detecting ulcers or bleeding, highlighting the potential of DL for identifying bleeding in CE images.17
The main current drawbacks of these systems are the offline design with mainly still images and very few videos. In addition, most studies are not conducted in real clinical practice. Besides, many of these studies include selection and spectrum biases. Thus, training using a vast number of images from different centers is necessary.
Additionally, little is known about the interaction between the AI machine and endoscopists. On one hand, the human brain may fall into biases such as over-reliance (blindly accepting AI decisions as true) or under-reliance. On the other hand, it is believed that the interaction between doctors and machines could create a symbiosis that increases the confidence of both the machine and the endoscopist in the diagnosis.18
Soon, multimodal AI is desired not only for endoscopic detection and diagnosis but also for clinical decision support, including pathological diagnosis and prognosis prediction. The goal could be to construct systems that can accurately predict treatment response, the risk of recurrence, and short-term and long-term prognosis.
THIRD SPACE ENDOSCOPY
Initially developed to achieve en bloc resection for gastrointestinal neoplasms without size limitations, endoscopic submucosal dissection (ESD) has evolved into third-space endoscopy in recent years enabling a wide range of therapeutic interventions not only within the submucosal layer but also within the muscular and subserosal layers.19
Since Inoue et al. performed the first peroral endoscopic myotomy (POEM) for achalasia in 2008, interest and potential indications for third-space endoscopy have increased. POEM has proven its effectiveness for the treatment of all types of achalasia as first line and salvage therapy and is now being applied to address other esophageal motility disorders.20, 21
POEM and other third-space procedures share a common principle, the establishment of a submucosal tunnel featuring a mucosal flap valve and the meticulous closure of the incision. The main derivatives are:
- POEM endoscopic fundoplication (POEM+F) an orifice transluminal endoscopic (NOTES) procedure could prevent gastroesophageal reflux disease in some cases. In this procedure, the peritoneal cavity is entered after POEM, and the anterior wall of the fundus is grasped and secured to the distal esophagus.22
- Peroral Endoscopic Tumor Resection (POET) for subepithelial lesions up to 40 mm has demonstrated high en-bloc resection rates (86.3%), with 18% of adverse events that could be managed conservatively.22
- During a Zenker-POEM (Z-POEM) the septum of the diverticulum, formed by the cricopharyngeal muscle, is dissected. It has shown a clinical success rate of 93% and an adverse event rate of 12%.21, 22
- Epiphrenic esophageal diverticulum has prompted the emergence of Diverticular-POEM (D-POEM) as a less invasive alternative to surgery. In D-POEM, septotomy of the diverticulum is performed. 19, 22
- Per-oral Endoscopic Tunneling for Restoration of the Esophagus (POETRE) is designed to manage long esophageal strictures that have limited treatment options. After creating the tunnel to access the stenotic area, the fibrotic scar tissue is dissected, followed by the placement of a fully covered self-expandable metal stent.21
- Gastric POEM (G-POEM) allows treating refractory gastroparesis and involves myotomy of the pyloric sphincter. However, long-term follow-up studies suggest that effectiveness may decrease up to 50% after the first year.21
- Per-rectal Endoscopic Myotomy (PREM) is a new technique for the endoscopic treatment of Hirschsprung's disease. Myotomy of the aganglionic segment is performed similarly to POEM. Nevertheless, more evidence is required before it can be integrated into daily clinical practice.22
Finally, defect closure is crucial during third-space endoscopy procedures. Innovative devices such as through-the-scope suture systems (TTSS) or endoscopic hand-suturing (EHS) systems are designed for addressing large defects. Initial studies have demonstrated high technical success rates (89-98%) along with clinical efficacy (97%) without notable adverse events. However, clinical applicability could be an issue, mainly due to the duration of the procedure with EHS devices. 23, 24, 25
SUMMARY
In conclusion, the convergence of telemedicine, artificial intelligence, and third-space endoscopy is transforming gastroenterology. These pillars promise to revolutionize patient care, diagnostics, and procedures. Telemedicine broadens healthcare access, AI enhances precision, and third-space endoscopy offers minimally invasive treatment. These innovations redefine gastroenterology, creating a patient-focused, technologically advanced healthcare system. Embracing these changes shapes the future of gastroenterological practice for patients and providers.
References
1. Ponsky JL, Strong AT. A History of Flexible Gastrointestinal Endoscopy. Surgical Clinics of North America. 2020 Dec 1;100(6):971–92.
2. Liu Z, Lv J, Dang Q, Liu L, Weng S, Wang L, et al. Engineering neoantigen vaccines to improve cancer personalized immunotherapy. Int J Biol Sci [Internet]. 2022;18(15):5607–23. Available from: https://www.ijbs.com/v18p5607.htm
3. Wang P, Berzin TM, Brown JRG, Bharadwaj S, Becq A, Xiao X, et al. Real-time automatic detection system increases colonoscopic polyp and adenoma detection rates: a prospective randomised controlled study. Gut [Internet]. 2019 Oct 1;68(10):1813. Available from: http://gut.bmj.com/content/68/10/1813.abstract
4. Edwards GC, Broman KK, Martin RL, Smalley WE, Smith LA, Snyder RA, et al. Virtual Colorectal Cancer Surveillance: Bringing Scope Rate to Target. J Am Coll Surg [Internet]. 2020 Aug 1 [cited 2023 Sep 30];231(2):257–66. Available from: https://journals.lww.com/journalacs/fulltext/2020/08000/virtual_colorectal_cancer_surveillance__bringing.10.aspx
5. López-Fernández A, Villacampa G, Grau E, Salinas M, Darder E, Carrasco E, et al. Patients’ and professionals’ perspective of non-in-person visits in hereditary cancer: predictors and impact of the COVID-19 pandemic. Genetics in Medicine [Internet]. 2021 Aug 1 [cited 2023 Sep 30];23(8):1450–7. Available from: http://www.gimjournal.org/article/S1098360021050632/fulltext
6. Munroe CA, Lin TY, Rouillard S, Fox J, Lee JK, Corley DA. Influence of Telemedicine-first Intervention on Patient Visit Choice, Postvisit Care, and Patient Satisfaction in Gastroenterology. Gastroenterology [Internet]. 2021 Feb 1 [cited 2023 Oct 8];160(3):929-931.e2. Available from: http://www.gastrojournal.org/article/S001650852035277X/fulltext
7. Fung BM, Markarian E, Serper M, Tabibian JH. Current Applications of Telemedicine in Gastroenterology. Vol. 117, American Journal of Gastroenterology. Wolters Kluwer Health; 2022. p. 1072–9.
8. de Jong MJ, Boonen A, van der Meulen-de Jong AE, Romberg-Camps MJ, van Bodegraven AA, Mahmmod N, et al. Cost-effectiveness of Telemedicine-directed Specialized vs Standard Care for Patients With Inflammatory Bowel Diseases in a Randomized Trial. Clinical Gastroenterology and Hepatology [Internet]. 2020 Jul 1 [cited 2023 Sep 30];18(8):1744–52. Available from: http://www.cghjournal.org/article/S1542356520305358/fulltext
9. Nashwan AJ, AbuJaber AA. Harnessing the Power of Large Language Models (LLMs) for Electronic Health Records (EHRs) Optimization. Cureus. 2023 Jul 29;
10. Okagawa Y, Abe S, Yamada M, Oda I, Saito Y. Artificial Intelligence in Endoscopy. Dig Dis Sci. 2022;67(5):1553–72.
11. Arribas J, Antonelli G, Frazzoni L, Fuccio L, Ebigbo A, van der Sommen F, et al. Standalone performance of artificial intelligence for upper GI neoplasia: a meta-analysis. Gut. 2020;
12. Meinikheim M, Messmann H, Ebigbo A. Role of artificial intelligence in diagnosing Barrett’s esophagus-related neoplasia. Clin Endosc. 2023;56(1):14–22.
13. Cho BJ, Bang CS, Lee JJ, Seo CW, Kim JH. Prediction of Submucosal Invasion for Gastric Neoplasms in Endoscopic Images Using Deep-Learning. J Clin Med. 2020;9(6).
14. Gong D, Wu L, Zhang J, Mu G, Shen L, Liu J, et al. Detection of colorectal adenomas with a real-time computer-aided system (ENDOANGEL): a randomised controlled study. Lancet Gastroenterol Hepatol. 2020;5(4):352–61.
15. Hassan C, Spadaccini M, Iannone A, Maselli R, Jovani M, Chandrasekar VT, et al. Performance of artificial intelligence in colonoscopy for adenoma and polyp detection: a systematic review and meta-analysis. Gastrointest Endosc. 2021;93(1):77-85.e6.
16. Repici A, Spadaccini M, Antonelli G, Correale L, Maselli R, Alessia Galtieri P, et al. Artificial intelligence and colonoscopy experience: lessons from two randomised trials. Gut [Internet]. 2022 [cited 2023 Oct 8];71:757–65. Available from: http://dx.doi.org/10.1136/gutjnl-2021-324471
17. Zhuang H, Bao A, Tan Y, Wang H, Xie Q, Qiu M, et al. Application and prospect of artificial intelligence in digestive endoscopy. Expert Rev Gastroenterol Hepatol. 2022;16(1):21–31.
18. Uche-Anya E, Anyane-Yeboa A, Berzin TM, Ghassemi M, May FP. Artificial intelligence in gastroenterology and hepatology: how to advance clinical practice while ensuring health equity. Gut. 2022;71(9):1909–15.
19. Inoue H, Navarro MJH, Shimamura Y, Tanabe M, Toshimori A. The Journey from Endoscopic Submucosal Dissection to Third Space Endoscopy. Vol. 33, Gastrointestinal Endoscopy Clinics of North America. W.B. Saunders; 2023. p. 1–6.
20. Vespa E, Pellegatta G, Chandrasekar VT, Spadaccini M, Patel H, Maselli R, et al. Long-term outcomes of peroral endoscopic myotomy for achalasia: a systematic review and meta-analysis. Vol. 55, Endoscopy. Georg Thieme Verlag; 2023. p. 167–75.
21. Nabi Z, Reddy DN. Submucosal endoscopy: the present and future. Clin Endosc. 2023 Jan 30;56(1):23–37.
22. Shimamura Y, Fujiyoshi Y, Fujiyoshi MRA, Inoue H. Evolving field of third-space endoscopy: Derivatives of peroral endoscopic myotomy. Vol. 35, Digestive Endoscopy. John Wiley and Sons Inc; 2023. p. 162–72.
23. Krishnan A, Shah-Khan SM, Hadi Y, Patel N, Thakkar S, Singh S. Endoscopic management of gastrointestinal wall defects, fistula closure, and stent fixation using through-the-scope tack and suture system. Endoscopy [Internet]. 2023 Oct 25 [cited 2023 Sep 18];55(8). Available from: https://pubmed.ncbi.nlm.nih.gov/36693419/
24. Mahmoud T, Wong Kee Song LM, Stavropoulos SN, Alansari TH, Ramberan H, Fukami N, et al. Initial multicenter experience using a novel endoscopic tack and suture system for challenging GI defect closure and stent fixation (with video). Gastrointest Endosc [Internet]. 2022 Feb 1 [cited 2023 Sep 18];95(2):373–82. Available from: http://www.giejournal.org/article/S0016510721017302/fulltext
25. Abe S, Saito Y, Tanaka Y, Ego M, Yanagisawa F, Kawashima K, et al. A novel endoscopic hand-suturing technique for defect closure after colorectal endoscopic submucosal dissection: A pilot study. Endoscopy. 2020 Sep 1;52(9):780–5.





